Microbiology & Infectious Diseases

Research Article:
IDWeek 2025: Hepatitis B Screening Gaps in HIV PrEP Care
Key Discussions, Breakthroughs, and Clinical Updates in HIV Prevention



Research Article:
IDWeek 2025: Hepatitis B Screening Gaps in HIV PrEP Care
Key Discussions, Breakthroughs, and Clinical Updates in HIV Prevention

Welcome 09 Foreword
Congress Review
10 IDWeek 2025 Annual Meeting Highlights, October 21st–24th, 2025
Congress Features
23 IDWeek 2025: Key Discussions and Breakthroughs
Majd Alsoubani
27 HIV Prevention: Clinical Updates from IDWeek 2025
Sara Hockney
Abstract Reviews
33 Evaluation of HIV Virologic Suppression Among Reincarcerated Individuals Within the Illinois Department of Corrections
Fouad et al.
35 Discontinuation Patterns and Virologic Failure Among Persons with HIV Receiving Long-Acting Injectable Cabotegravir-Rilpivirine
Antiretroviral Therapy
Hockney et al.
37 Screening and Incidence of Sexually Transmitted Infections Among Persons Living with HIV in the Illinois Department of Corrections
Schreiber et al.
39 Incidence of Mycobacterium tuberculosis Screening and Detection in People Living with HIV in Custody Within the Illinois Department of Corrections
Stickler et al.
41 Performance of Sputa Gram Stain for the Evaluation of Staphylococcus aureus and Pseudomonas aeruginosa Pneumonia, as Compared to Standard Culture and Multiplex PCR
Sera et al.
44 Blood Culture Stewardship Efforts at a Comprehensive Cancer Center Reduced Isolation of Skin Flora Contaminants Without Compromising Patient Care
Borjan et al.
47 Development of a Severity Scoring System for Mycoplasma pneumoniae Infection
Kelly et al.
50 Descriptive Analysis of Positive Strongyloides Serology Across Three Academic Medical Centers
Nagarakanti et al.
52 Incidence and Cumulative Risk Factors for Prolonged Corrected QT Interval in Patients with Liver Cirrhosis
Receiving Fluoroquinolone Prophylaxis
Pustorino et al.
Congress Interview
55 Barbara Trautner Interview
59 Tina Tan Features
61 Tick, Tick, Boom! An Update on Tickborne Infections in the United States
Karen C. Bloch
70 Cutaneous Histoplasmosis in Patients with HIV: Brief Review
Cano et al. Articles
76 Editor's Pick: Guideline Adherence to Hepatitis B Virus Screening and Vaccination in Patients Prescribed HIV Oral Pre-exposure Prophylaxis
Ahwad and Badowski
85 Challenges in HIV Monitoring: Insights from Cluster of Differentiation 4 Variability in Resource-Limited Settings
Galo Guillermo Farfán Cano
92 Impact of Lactobacillus Rhamnosus GG on Weight Loss in PostBariatric Surgery Patients: A Randomized, Double-Blind Clinical Trial
Nasir et al.
102 Evaluation of Kabul University Students’ Attitude and Knowledge About Antibiotics and Their Use
Dawlatpoor et al.


Dr Shira Doron
Tufts Medical Center, Boston, Massachusetts, USA
Dr Shira Doron is the Chief Infection Control Officer for the Tufts Medicine Health System and the Hospital Epidemiologist for Tufts Medical Center, where she is an Infectious Disease physician. She is a Professor of Medicine at Tufts University School of Medicine. Dr Doron is a member of the Infectious Diseases Society of America’s (IDSA) Practice and Quality Committee and the immediate past chair of the society’s Antimicrobial Stewardship Centers of Excellence subcommittee. She is a long-time consultant to the Massachusetts Department of Public Health in the area of antimicrobial resistance prevention, focusing on long-term care facilities. She was a member of Governor Charlie Baker’s Medical Advisory Board during the COVID-19 pandemic. She is also an elected member of the Wellesley Board of Health.








Dr Lisa Akhtar
Ann & Robert H. Lurie
Children’s Hospital of Chicago, Illinois, USA
Dr Michael Angarone
Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA
Dr Shweta Anjan
University of Miami Miller School of Medicine, Florida, USA
Dr Karen C. Bloch
Vanderbilt University Medical Center, Nashville, Tennessee, USA








Dr Syra Madad
New York City Health and Hospitals, USA
Dr L. Silvia Munoz Price
Emerald Coast Infectious Diseases, Fort Walton Beach, Florida, USA
Dr Sandhya Nagarakanti
Mayo Clinic-Arizona, Phoenix, USA
Dr Maurice Policar
New York City Health and Hospitals/Elmhurst, USA
AMJ Microbiology & Infectious Diseases is an open-access, peer-reviewed eJournal committed to helping elevate the quality of healthcare by publishing high quality content on all aspects of microbiology and infectious diseases.
The journal is published annually, 6 weeks after the IDWeek 2025, and features highlights from this congress, alongside interviews with experts in the field, reviews of abstracts presented at the congress, as well as in-depth features on congress sessions. Additionally, this journal covers advances within the clinical and pharmaceutical arenas by publishing sponsored content from congress symposia, which is of high educational value for healthcare professionals. This undergoes rigorous quality control checks by independent experts and the in-house editorial team. AMJ Microbiology & Infectious Diseases also publishes peerreviewed research papers, review articles, and case reports in the field. In addition, the journal welcomes the submission of features and opinion pieces intended to create a discussion around key topics in the field and broaden readers’ professional interests. AMJ Microbiology & Infectious Diseases is managed by a dedicated editorial team that adheres to a rigorous double-blind peer-review process, maintains high standards of copy editing, and ensures timely publication.
Our focus is on research that is relevant to all healthcare professionals in microbiology and infectious diseases. We do not publish veterinary science papers or laboratory studies not linked to patient outcomes. We have a particular interest in topical studies that advance research and inform of coming trends affecting clinical practice in the respiratory field. is an open access, peer-reviewed eJournal committed to helping elevate the quality of practices in microbiology and infectious diseases globally by informing healthcare professionals on the latest research in the field.
Further details on coverage can be found here: www.emjreviews.com/en-us/
Editorial Expertise
AMJ is supported by various levels of expertise:
• Guidance from an Editorial Board consisting of leading authorities from a wide variety of disciplines.
• Invited contributors who are recognised authorities in their respective fields.
• Peer review, which is conducted by expert reviewers who are invited by the Editorial team and appointed based on their knowledge of a specific topic.
• An experienced team of editors and technical editors.
Peer Review
On submission, all articles are assessed by the editorial team to determine their suitability for the journal and appropriateness for peer review.
Editorial staff, following consultation with either a member of the Editorial Board or the author(s) if necessary, identify three appropriate reviewers, who are selected based on their specialist knowledge in the relevant area.
All peer review is double blind.Following review, papers are either accepted without modification, returned to the author(s) to incorporate required changes, or rejected.
Editorial staff have final discretion over any proposed amendments.
We welcome contributions from professionals, consultants, academics, and industry leaders on relevant and topical subjects. We seek papers with the most current, interesting, and relevant information in each therapeutic area and accept original research, review articles, case reports, and features.
We are always keen to hear from healthcare professionals wishing to discuss potential submissions, please email: editorial@americanmedicaljournal.com
To submit a paper, use our online submission site: www.editorialmanager.com/e-m-j
Submission details can be found through our website: www.emjreviews.com/contributors/authors
All articles included in AMJ are available as reprints (minimum order 1,000). Please contact hello@emjreviews.com if you would like to order reprints.
Distribution and Readership
AMJ is distributed globally through controlled circulation to healthcare professionals in the relevant fields.
Indexing and Availability
EMJ is indexed on DOAJ, the Royal Society of Medicine, and Google Scholar®; selected articles are indexed in PubMed Central®
AMJ is available through the websites of our leading partners and collaborating societies. AMJ journals are all available via our website: www.emjreviews.com/en-us/
Open Access
This is an open-access journal in accordance with the Creative Commons Attribution-Non Commercial 4.0 (CC BY-NC 4.0) license.
Congress Notice
Staff members attend medical congresses as reporters when required.
This Publication
Launch Date: November 2025 Frequency: Annually Online ISSN: 2977-4055
All information obtained by AMJ and each of the contributions from various sources is as current and accurate as possible. However, due to human or mechanical errors, AMJ and the contributors cannot guarantee the accuracy, adequacy, or completeness of any information, and cannot be held responsible for any errors or omissions. EMJ is completely independent of the review event (IDWeek 2025) and the use of the organisations does not constitute endorsement or media partnership in any form whatsoever. The cover photo is of Atlanta, Georgia, USA, the location of IDWeek 2025.
Front cover and contents photograph: Atlanta, Georgia © Jose Luis Stephens / stock.adobe.com



We provoke conversation around healthcare trends and innovation - we also create engaging educational content for healthcare professionals. Join us for regular conversations with physician & entrepreneur, Jonathan Sackier. Listen Now
Editorial
Helena Bradbury, Ada Enesco, Noémie Fouarge, Niamh Holmes, Sarah Jahncke, Bertie Pearcey, Alena Sofieva, Katrina Thornber, Aleksandra Zurowska
Design
Dillon Benn Grove, Shanjok Gurung, Tamara Kondolomo, Owen Silcox, Helena Spicer, Fabio van Paris
Managing Editor Darcy Richards
Design Manager
Stacey White
Head of Marketing
Stephanie Corbett
Creative Director
Tim Uden
Editorial Director
Andrea Charles
Vice President of Content
Anaya Malik
Vice President of Customer Success
Alexander Skedd
Vice President of Business Development
Robert Hancox
Chief Executive Officer
Justin Levett
Chief Commercial Officer
Dan Healy
Founder and Chairman
Spencer Gore
Contact us
Dear Readers,
Welcome to the latest issue of AMJ Microbiology & Infectious Diseases. This publication brings together the latest insights and trends transforming infectious diseases today, combining expert insights, IDWeek 2025 Annual Meeting coverage, and communitydriven learning.
With the dust now settled after IDWeek 2025, the AMJ team has compiled the most exciting takeaways. From evolving strategies in HIV prevention to stewardship innovations, this issue captures key developments in modern clinical practice and real-world challenges that shaped the themes of the gathering.
Our interviews shine a spotlight on two leaders offering firsthand insights on the new urinary tract infection guidelines presented at IDWeek 2025, as well as the challenges of miscommunication around vaccine hesitancy and the importance of trust. The featured articles uncover essential gaps in and opportunities to optimise preventative care in HIV, and how gut-targeted science continues to influence infectious diseases.
Readers will find our congress review packed with award-winning abstracts presented at IDWeek 2025 and discover the emerging research shaping treatment decisions.
My sincere thanks go to the Editorial Board, authors, peer reviewers, presenters, interviewees, and production team, as well as our audience, for your continued trust, intrigue, and contributions.

Editorial enquiries: editorial@americanmedicaljournal.com
Sales opportunities: info@americanmedicaljournal.com
Permissions and copyright: accountsreceivable@emjreviews.com
Anaya Malik Vice President of Content
Reprints: info@emjreviews.com
Media enquiries: marketing@emjreviews.com
The contents of this publication highlight the ongoing contributions from researchers and clinicians dedicated to understanding the complexities of infectious diseases, leading with the breakthroughs from this year’s IDWeek Annual Meeting. We incorporate key developments in modern clinical practice, including new perspectives on long-acting antiretroviral therapy, refinements in screening approaches for high-risk populations, and emerging research in antimicrobial resistance.
The featured articles span a spectrum of microbial and host interactions. The evolving issue of antimicrobial resistance is explored, with a deep dive into how the roles of telehealth, point-of-care testing, and inpatient stewardship are becoming essential to guiding treatment decisions and streamlining care. Notably, the contributions from IDWeek 2025, including clinical updates on HIV treatment and the impact of Mycobacterium tuberculosis screening in correctional settings, provide timely insights that reinforce the importance of continuous innovation in patient care.
The issue also sheds light on critical topics in the treatment of bacterial infections, ranging from the effectiveness of Lactobacillus rhamnosus GG in post-bariatric surgery patients to new insights on the molecular mechanisms behind Mycoplasma pneumoniae infections, and improving the clinical care


of patients receiving HIV pre-exposure prophylaxis with a focus on adhering to established guidelines.
As we look toward the future of infectious disease management, this issue underscores the importance of innovation, collaboration, and adherence to evidence-based practices.
I extend my gratitude to all contributors, authors, and reviewers for their continued dedication to the field.
The contents of this publication highlight the ongoing contributions from researchers and clinicians dedicated to understanding the complexities of infectious diseases
Their work ensures that AMJ Microbiology & Infectious Diseases remains at the forefront of advancing knowledge and fostering discussions that drive healthcare improvements.
Please engage and join us as we continue to enhance patient care and empower excellence in medicine.
Shira Doron Chief Infection Control Officer, Tufts Medicine; Professor of Medicine, Tufts University School of Medicine, Boston, Massachusetts, USA

The IDWeek 2025 Annual Meeting showcased pivotal advances in infectious diseases, from realworld vaccine data to novel therapeutic approaches
Location: Atlanta, Georgia, USA
Date: October 19th–22nd, 2025
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:10-22. https://doi.org/10.33590/microbiolinfectdisam/UVIO7409
THE IDWEEK 2025 Annual Meeting showcased pivotal advances in infectious diseases, from real-world vaccine data and innovative stewardship strategies to novel therapeutic approaches reshaping clinical care. This year’s highlights reflect a continued emphasis on optimizing antimicrobial use, improving outcomes for vulnerable populations, and translating evidence into practice.

A NEW real-world study presented at IDWeek 2025 reports that the recombinant zoster vaccine is associated with significantly reduced risks of death and major cardiovascular events in people living with HIV (PLWH). The analysis, conducted by researchers at Case Western Reserve University School of Medicine, Cleveland, Ohio, USA, provides new evidence supporting broader health benefits of zoster vaccination in this high-risk population.1
Chronic immune activation in PLWH contributes to an elevated risk of cardiovascular and neurodegenerative conditions, while herpes zoster infection can further intensify these complications. Using the TriNetX Analytics Network (TriNetX, Cambridge, Massachusetts, USA), investigators performed a retrospective matched cohort study including 3,146 adults aged ≥50 years (mean age: 58.4 years) with HIV and no prior herpes zoster diagnosis. Participants were divided by vaccination status and matched 1:1 on demographics, antiretroviral therapy regimen, comorbidities, psychiatric history, and prior vaccine exposures to ensure balanced comparison groups. After matching, the cohorts were well balanced across key variables.
During a follow-up period ranging from 90 days–7 years (median: 2.89 years in the vaccinated group and 2.78 years in the unvaccinated group), prior zoster vaccination was associated with a 47% lower hazard of all-cause mortality (hazard ratio [HR]: 0.534; 95% CI: 0.380–0.749; p=0.0002) and a 39% reduction in major adverse cardiovascular
These findings reinforce the importance of comprehensive vaccination strategies as part of holistic care for PLWH
events (HR: 0.614; 95% CI: 0.481–0.783; p=0.0001). A trend toward reduced dementia risk was observed among vaccinated individuals (HR: 0.559; 95% CI: 0.237–1.32; p=0.1783), though this result did not reach statistical significance. No significant differences were found in psychiatric morbidity or Parkinsonism between the groups.
The study demonstrates that zoster vaccination in PLWH is associated with improved long-term survival and lower cardiovascular risk, suggesting potential systemic benefits beyond the prevention of shingles. These findings reinforce the importance of comprehensive vaccination strategies as part of holistic care for PLWH.
Infections remain a leading cause of morbidity and mortality after kidney transplantation, and UTIs are particularly common in the early months post-surgery. The study examined whether daily cleansing of the surgical site and perineum with 2% chlorhexidine gluconate cloths for 3 months following discharge could reduce infection-related complications. Participants received decolonization kits at discharge, with subsequent monthly kits mailed to their homes, accompanied by detailed instructions from transplant nurses.
The study included 517 adult kidney transplant recipients, of whom 94 received the intervention and 423 served as controls, drawn from the same intervention period and a 2-year pre-intervention period. Outcomes were evaluated using Kaplan–Meier survival analysis and Cox proportional hazards models adjusted for baseline differences.
The study included adult kidney transplant recipients, of whom received the intervention and served as controls
A PRAGMATIC quality improvement study presented at IDWeek 2025 found that post-discharge home decolonization significantly reduced urinary tract infections (UTI) and graft failure in kidney transplant recipients, with a trend toward lower mortality. Conducted by researchers at the University of California, Irvine, USA, the study evaluated a simple, safe, and cost-effective strategy to prevent early post-transplant complications.2 517 94 423
Participants received decolonization kits at discharge, with subsequent monthly kits mailed to their homes
Recipients of the home decolonization intervention experienced significantly lower rates of UTI (19.2% versus 34.8%) and graft failure (0.0% versus 2.8%) compared to non-participants. Deaths were fewer in the intervention group (2.1% versus 6.2%), though this difference did not reach statistical significance. Kaplan–Meier analysis demonstrated higher bacteriuriafree survival at 30, 60, and 90 days posttransplant among participants (88.3%, 83.0%, and 80.9%, respectively) compared with controls (74.5%, 67.1%, and 65.3%; log-rank p<0.004). Adjusted analyses confirmed a significantly lower risk of UTI for participants (adjusted hazard ratio: 0.56; 95% CI: 0.30–0.82; p<0.004). Adverse events were rare, occurring in approximately 1% of participants.
These findings indicate that post-discharge chlorhexidine gluconate bathing is an effective, low-cost approach to reducing infection-related complications after kidney transplantation. The intervention provides a resistance-sparing alternative to antibiotics, offering a practical strategy to improve outcomes and support graft survival in transplant recipients.
A STUDY presented at IDWeek 2025 found that shorter courses of antibiotic therapy were as safe and effective as longer regimens following the removal of infected cardiac implantable electronic devices (CIED). The findings may help refine treatment practices and reduce unnecessary antibiotic use.3
Infections involving CIEDs, such as pacemakers and defibrillators, are the most common reason for lead extraction. While complete removal of the infected device is standard practice, the optimal duration of antibiotic treatment after extraction has not been clearly defined. This study is the first to evaluate how antimicrobial duration affects clinical outcomes in this setting.
Researchers reviewed 747 patient cases between June 2013–December 2023, identifying 79 patients who met inclusion criteria for extraction due to bacteremia or lead-associated infection. Patients were grouped by antibiotic duration of ≤2 weeks or >2 weeks. Baseline characteristics were similar between cohorts. The median duration of antibiotic therapy was 12.6 days for patients receiving ≤2 weeks of antibiotics and 38.6 days for those receiving >2 weeks of antibiotics.
Kaplan–Meier survival analysis showed no significant difference in survival between
the two groups (hazard ratio: 0.693; 95% CI: 0.085–5.652; p=0.438). There were no significant differences in recurrent bacteremia (7% versus 6%; p=0.952), infectious complications (27% versus 30%; p=0.817), hospital length of stay (mean: 9.9 versus 13.3 days; p=0.360), postoperative ICU disposition (0% versus 17%; p=0.112), or rates of cardiac arrest (7% versus 5%; p=0.577).
Relapse or recurrence occurred in five patients, all of whom had infections caused by Staphylococcus aureus (n=3) or Serratia species (n=2) and had either a left ventricular assist device or a valve replacement.
The findings indicate that shorter durations of antibiotic therapy, defined as 2 weeks or less, after CIED lead extraction are not associated with increased mortality or higher rates of recurrent bacteremia. Further studies are needed to determine optimal antibiotic duration and identify risk factors for recurrence in this patient population.
A STUDY from the National Taiwan University Hospital, Taipei, Taiwan, presented at IDWeek 2025, reported high mortality rates among patients with bloodstream infections caused by daptomycin-resistant vancomycin-resistant Enterococcus. The research revealed several key prognostic factors and treatment implications for this emerging antimicrobial threat.4
From 2010–2024, investigators analyzed 2,230 vancomycin-resistant Enterococcus bloodstream infection episodes, identifying 120 cases that met the criteria for daptomycin resistance. The median patient age was 67.3 years, with 57.5% being male. Primary bloodstream (45.8%) and urinary tract infections (43.3%) accounted for most cases.
The overall 28-day mortality rate reached 47.5%. Multivariable analysis showed that a higher Charlson Comorbidity Index (CCI;
adjusted odds ratio [aOR]: 1.30; p=0.02), elevated Pitt bacteremia score (aOR: 1.35; p<0.01), and lower platelet count (aOR: 0.98; p<0.01) were independently linked to increased mortality.
Of the 120 patients, 101 (84.1%) received daptomycin while 19 (15.8%) received linezolid. Patients treated with moderate daptomycin doses (8–11 mg/kg) had higher mortality (aOR: 3.30; p=0.04) than those receiving doses greater than 11 mg/kg. No significant difference in survival was found between high-dose daptomycin and linezolid (aOR: 2.02; p=0.39).
Recurrent bacteremia occurred in about 9% of cases, showing no major variation between treatment groups (15.6% versus 18.2%; p=0.82).
Researchers concluded that high-dose daptomycin, at or above 11 mg/kg, may offer comparable outcomes to linezolid despite laboratory indications of resistance. They emphasized that optimizing dosing and managing underlying risk factors are essential to improving patient survival.
Patients treated with moderate daptomycin doses (8–11 mg/kg) had higher mortality (aOR: 3.30; p=0.04) than those receiving doses greater than 11 mg/kg
USING the TriNetX Global Collaborative Network (TriNetX, Cambridge, Massachusetts, USA), a new study presented at IDWeek 2025 has found that the use of dalbavancin in people who inject drugs (PWID) with Staphylococcus aureus infective endocarditis (IE) is associated with lower mortality and fewer adverse events compared with standard intravenous (IV) antibiotic therapy, supporting its role as a safer and more practical treatment option in this high-risk population.5
Treating IE in PWID remains a major clinical challenge. Prolonged IV antibiotic courses are often difficult to complete due to social, behavioral, and logistical barriers. Discharging patients with peripherally inserted central catheters carries risks of line misuse, reinfection, and treatment failure. Dalbavancin, a long-acting lipoglycopeptide that allows for infrequent dosing, is an alternative for those unable to safely receive outpatient parenteral antibiotic therapy. However, its real-world effectiveness relative to conventional regimens has not been clearly established.
The research team conducted a retrospective, propensity score-matched cohort study of adults aged 18 years and older with S. aureus IE and a history of substance use. Patients treated with dalbavancin (n=288) were matched 1:1 with those receiving standard IV antibiotics, including vancomycin, daptomycin, cefazolin, linezolid, or nafcillin (n=288), based on age and sex. The primary outcome was 1-year all-
6.9 %
At 1 year, mortality was significantly lower with dalbavancin at with standard IV antibiotics
compared to
15.6 %
cause mortality, while secondary endpoints included recurrent bacteremia, acute kidney injury, Clostridioides difficile infection, and rash. The median patient age was 38 years, and 49.3% were male. At 1 year, mortality was significantly lower with dalbavancin at 6.9%, compared to 15.6% with standard IV antibiotics, with a risk difference of –8.7 percentage points (95% CI: -13.8–-3.6; hazard ratio [HR]: 0.44; p=0.002). Dalbavancin was also associated with reduced acute kidney injury (11.4% versus 30.6%; HR: 0.32; p<0.001), rash (6.9% versus 12.8%; HR: 0.52; p=0.015), and recurrent bacteremia (52.8% versus 60.4%; HR: 0.36; p=0.001).
These findings indicate that dalbavancin may provide an effective and safer alternative to traditional IV therapy for S. aureus IE in PWID. Incorporating dalbavancin into clinical pathways could improve adherence, reduce hospital readmissions, and lessen complications related to IV access.
MMBV assists clinicians in distinguishing bacterial from viral infections
RESEARCH presented at IDWeek 2025 has demonstrated that integration of the MeMed BV® (MMBV; MeMed, Tirat Carmel, Israel) host-response test into clinical decision-making in urgent care centers (UCC) is associated with improved patient outcomes, including reduced hospitalization rates within 7 days of discharge.6
Inappropriate antibiotic prescribing remains a significant challenge in UCCs, where diagnostic uncertainty is often high due to limited consultation time and restricted access to diagnostic tools. MMBV, an FDAcleared test that evaluates the host immune response by combining levels of three immune proteins into a bacterial likelihood score, assists clinicians in distinguishing bacterial from viral infections. The test demonstrates a negative predictive value greater than 98%, making it a reliable aid in guiding antibiotic prescribing decisions and enhancing antimicrobial stewardship efforts.
This retrospective analysis assessed realworld data from 3,758 adult patients tested with MMBV during visits to 10 UCCs between April–December 2022. Of these, 59.3% were female and the median age was 42 years (interquartile range: 31–58). Bacterial results were reported in 858 patients (22.8%), viral in 2,404 (64.0%), and equivocal in 496 (13.2%). It was also revealed that patients with bacterial MMBV results were older
(median: 51 versus 39 years) and more frequently diagnosed with lower respiratory tract infections (29.6% versus 9.4%). Among those with bacterial results, antibiotic treatment was associated with significantly fewer hospitalizations within 7 days (7.3% versus 36.1%; p<0.001). For viral results, withholding antibiotics correlated with a lower rate of lower respiratory tract infection diagnosis (4.2% versus 29.5%) and fewer hospitalizations (2.5% versus 5.3%; p=0.003).
This study shows that the incorporation of MMBV into urgent care workflows appears to improve clinical outcomes while supporting appropriate antibiotic prescribing. By providing clinicians with timely hostresponse insights, MMBV may reduce unnecessary antibiotic exposure, hospital admissions, and associated healthcare costs. Further prospective studies could evaluate its integration into broader infection management protocols and explore long-term impacts on antimicrobial resistance trends.
PERSONS experiencing homelessness (PEH) face a disproportionately high burden of chronic hepatitis C virus (HCV) infection and often encounter significant barriers to diagnosis, follow-up, and treatment. Conventional point-of-care (POC) HCV antibody tests only indicate prior exposure rather than active infection, creating delays in linking individuals to care. The Cepheid Xpert HCV test (Cepheid, Sunnyvale, California, USA) offers a rapid method to detect active HCV RNA, allowing health providers to diagnose infection and begin treatment more quickly. A recent study, presented at IDWeek 2025, evaluated the use of the Xpert HCV test within a street medicine model to improve HCV care among PEH.7
From November 2024–February 2025, weekly street medicine outreach runs were conducted. Individuals were offered testing using a finger-stick blood sample, which was analyzed for both HCV antibody and active HCV RNA on a POC Cepheid Xpress system (Cepheid, Sunnyvale, California, USA). Those who tested positive for HCV RNA received confirmatory bloodwork, including viral load and genotype testing, and were assessed for eligibility for simplified treatment with directacting antivirals. Eligible participants were provided medication directly on a weekly or biweekly basis, accompanied by adherence support. Viral load was measured again at the end of treatment to assess response.
Among the individuals tested, 20 were HCV antibody-positive and 13 had active infection. Twelve were able to complete additional bloodwork, and all met guideline-based criteria for simplified direct-acting antiviral therapy. Nine patients began treatment, four completed therapy, and three achieved undetectable viral RNA at the end of treatment. No HIV or hepatitis B co-infection was observed. The average time from testing to initiation of treatment was approximately 20 days.
These findings show that rapid RNA-based POC testing can successfully identify active infection and facilitate timely treatment among PEH. Integrating such testing into
street medicine can meaningfully advance HCV elimination efforts in highly marginalized populations.

A RECENT study, presented at IDWeek 2025, described how a national shortage of blood culture (BCx) supplies prompted the implementation of targeted mitigation measures at a large academic medical center.8
BCx contamination rates also decreased from 1.96% to 1.66%, suggesting improved diagnostic stewardship
These measures aimed to decrease unnecessary BCx use while evaluating potential effects on antibiotic prescribing practices, contamination rates, and key outcomes related to sepsis management. Clinicians were supported through electronic clinical decision support tools that offered soft stops on repeat cultures, required acknowledgement for repeat BCx performed within 48 hours, and suggested alternative testing options. Education efforts were directed across the institution, with particular focus on areas with historically high BCx utilization, including the emergency department and oncology units.
This retrospective pre- and post-intervention study compared data from a pre-shortage period (July 2023–May 2024) to the shortage period (July 2024–November 2024). The primary outcome was antibiotic days of therapy per 1,000 patient-days. Secondary outcomes included the number of blood cultures obtained, sepsis core measure performance, length of stay, inpatient mortality, and rates of blood culture contamination. Statistical comparisons were performed using the Wilcoxon rank sum test.
BCx utilization declined significantly during the shortage, decreasing from a median of 1,846 to 1,205 cultures. Despite concerns that reduced culture availability might lead to increased empiric antibiotic use, overall antibiotic consumption remained stable. Notably, in the emergency department, use of vancomycin, cefepime, and ceftriaxone decreased significantly. Patient outcomes were not negatively affected: inpatient mortality remained unchanged, and length of stay showed a modest reduction. BCx contamination rates also decreased from 1.96% to 1.66%, suggesting improved diagnostic stewardship.
These findings indicate that targeted mitigation strategies can effectively reduce BCx use during shortages without compromising antibiotic stewardship or sepsis care. The reductions in selected antibiotics and contamination rates further support careful culture utilization as a component of high-quality clinical practice.
These findings indicate that targeted mitigation strategies can effectively reduce BCx use during shortages
NEW RESEARCH presented at IDWeek 2025 has provided the first largescale, real-world evidence of how the 2021 Infectious Diseases Society of America (IDSA)/Society for Healthcare Epidemiology of America (SHEA) Clostridioides difficile infection (CDI) treatment guidelines have reshaped clinical practice, improved patient outcomes, and influenced healthcare costs across the USA.9
The 2021 guideline update recommended fidaxomicin over vancomycin as the preferred first-line therapy for both initial and recurrent CDI, and bezlotoxumab as an adjuvant treatment for high-risk patients. In this new analysis, Angela Wu and colleagues from the Baylor College of Medicine, Houston, Texas, USA, examined over 1.2 million CDI encounters from 2018–2024 using the Epic Cosmos database, comparing treatment patterns and outcomes before and after the guideline release in June 2021.
Following implementation, the researchers observed clear shifts in prescribing behavior: fidaxomicin use tripled from 3.1% to 9.6%, vancomycin use rose slightly, and bezlotoxumab prescriptions increased fivefold, while metronidazole use dropped by nearly half. Importantly, these changes coincided with a measurable clinical benefit. The odds of 30-day CDI recurrence fell by 4% immediately after the guideline update (odds ratio: 0.96; 95% CI: 0.94–0.97; p=0.001), and
recurrence trends flattened in the months that followed.
While hospital length of stay increased briefly after implementation, it declined significantly over time as practices stabilized. However, the financial impact was notable: total monthly CDI-related costs surged by nearly 20 million USD in the immediate postguideline period, largely due to the higher costs of fidaxomicin and bezlotoxumab. Despite this, the study found no ongoing upward cost trend in the following years.
The authors concluded that adoption of the 2021 IDSA/SHEA CDI guidelines has led to fewer recurrences and improved care outcomes, but also emphasized the importance of addressing the economic burden associated with newer, higher-cost therapies. These findings underscore the balance between evidence-based advances and cost sustainability in infectious disease management.
THE LATEST research presented at IDWeek 2025 suggests that shorter antibiotic courses may be just as effective, and potentially safer, for hospitalized patients with urinary tract infections (UTI) under the new Infectious Diseases Society of America (IDSA) definition of uncomplicated UTI.10
The forthcoming IDSA complicated UTI guidelines redefine complicated UTI as infections extending beyond the bladder, meaning that many patients previously classified as having complicated infections would now fall under the uncomplicated category. This reclassification expands the group of patients eligible for shorter antibiotic treatment durations, a shift that could significantly impact prescribing practices and antimicrobial stewardship nationwide.

Researchers analyzed data from 68 hospitals in Michigan, USA, collected between November 2021–November 2024, encompassing 13,784 hospitalized patients with UTI, of whom 1,854 met the new uncomplicated UTI definition. Using a target trial emulation framework and rigorous statistical adjustments, the study compared outcomes between patients receiving short (3–5 day) and long (6–14 day) antibiotic regimens.
Results showed no significant difference in 30-day recurrence rates between the two treatment durations (odds ratio: 0.73; 95% CI: 0.45–1.17). However, patients treated with shorter courses experienced fewer antibioticrelated adverse events (odds ratio: 0.33; 95% CI: 0.12–0.95). Common characteristics, including comorbidities and infection severity, were similar between groups, and ceftriaxone was the most frequently prescribed empiric antibiotic.
The findings reinforce growing evidence that short-course antibiotic therapy is both effective and safer for patients with UTIs that do not extend beyond the bladder. By minimizing antibiotic exposure without compromising efficacy, this approach could help curb adverse drug effects and reduce the risk of antimicrobial resistance.
The authors concluded that the results provide strong real-world support for shorter antibiotic durations in patients meeting the new uncomplicated UTI definition, aligning with the IDSA’s evolving recommendations for optimized, evidence-based antimicrobial use.
1. Dehghani A et al. Zoster vaccination in people living with HIV is associated with reduced mortality and cardiovascular risk: a real-world matched cohort study. Oral abstract P-402. IDWeek, October 19-22, 2025.
2. Nam HH et al. Home decolonization to decrease UTI, graft failure, and death after renal transplantation (PROTEKT: PROTEction after Kidney Transplant): a pragmatic quality improvement study. Oral abstract P-295. IDWeek, October 19-22, 2025.
3. Xiao EY et al. Shorter duration of antimicrobial therapy is noninferior for cardiovascular implantable electronic device associated systemic infections. Abstract 453. IDWeek, October 19-22, 2025.
4. Lin WT et al. High dose daptomycin shows non-inferior outcome compared to linezolid in patients with daptomycin and vancomycin resistant enterocci bloodstream infection. Abstract 367. IDWeek, October 19-22, 2025.
5. Ssentongo P et al. Comparative effectiveness of dalbavancin versus standard therapy for staphylococcus aureus endocarditis in people who inject drugs: a retrospective, propensity-matched cohort study using real-world data. Abstract 451. IDWeek, October 19-22, 2025.
6. David SSB et al. Host-response testing to guide antibiotic prescription: association between MeMed BV® results and clinical outcomes in an urgent care network. Abstract 148. IDWeek, October 19-22, 2025.
7. Crooker KG et al. Implementation of novel point-of-care hepatitis C RNA platform and clinical characteristics of treatment in persons experiencing homelessness in Detroit, Michigan. Abstract 199. IDWeek, October 19-22, 2025.
8. Kusnik N et al. Intended and unintended consequences of a blood culture bottle shortage. Abstract 432. IDWeek, October 19-22, 2025.
9. Wu et al. Real-world impact of the 2021 IDSA/SHEA CDI guidelines: shifts in treatment, outcomes, and healthcare costs. Abstract 194. IDWeek, October 19-22, 2025.
10. Steinberger M et al. A target trial emulation of short vs long antibiotic duration for the new definition of uncomplicated UTI. Abstract 376. IDWeek, October 19-22, 2025.
Author: *Majd Alsoubani1,2
1. Division of Geographic Medicine and Infectious Diseases, Department of Medicine, Tufts Medical Center, Boston, Massachusetts, USA
2. The Stuart B. Levy Center for the Integrated Management of Antimicrobial Resistance, School of Medicine, Tufts University, Boston, Massachusetts, USA
*Correspondence to Majd.Alsoubani@tuftsmedicine.org
Disclosure: Alsoubani has received grant funding from Carb-X as a principal investigator.
Keywords: Health access, HIV prevention, IDWeek 2025, public health.
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:23-26. https://doi.org/10.33590/microbiolinfectdisam/RVPF8742
ATLANTA, Georgia, USA, homebase for the CDC and a fitting stage for public health discourse, welcomed thousands of clinicians, scientists, pharmacists, and trainees for IDWeek 2025. The 4-day meeting combined late-breakers, pragmatic debates, hands-on workshops, and the ever-busy BugHub stages.
Javier Muñoz, actor and advocate, opened IDWeek 2025 with a deeply personal reflection on living with HIV. His story brought data to life, grounding science in real human experience and reminding everyone that innovation only matters when it reaches the people who need it most.
The Opening Plenary embraced the spirit of IDWeek’s host city, with speakers highlighting the resilience and dedication of public health professionals at the CDC and across partner organizations. The theme, 'Reflection and renewal: advancing public health in challenging times', struck a balance between realism and optimism. It acknowledged workforce fatigue and funding challenges while emphasizing
readiness, stronger communication, and the power of implementation science to drive progress.
From there, speakers emphasized that preparedness is not just about responding to the next emergency, but a constant commitment to building surveillance, communications, and clinical capacity. They called for continued vigilance in vaccine confidence and safety, practical approaches to antimicrobial stewardship, and a sustained focus on equity and access to care.
IDWeek’s awards underscored the community’s depth and breadth. The Infectious Diseases Society of America (IDSA) recognized leaders across clinical care, research, mentorship, and public health. Awardees included John Boyce, J.M.
Boyce Consulting, New Haven, Connecticut, USA, who received the Society for Healthcare Epidemiology of America (SHEA) award for his career-long impact on hand hygiene research and preventing healthcare-associated infections; Walter Orenstein, Emory University, Atlanta, Georgia, USA, who was awarded the Pediatric Infectious Diseases Society (PIDS) award recognizing decades of work shaping U.S. and global immunization policy; and David Ha, Stanford, Menlo Park, California, USA, who accepted the Society of Infectious Diseases Pharmacists (SIDP) 2025 Outstanding Clinician Award. The IDSA Society Citation Award was presented to the IDSA Chief Executive Officer, Christopher D. Busky, Arlington, Virginia, USA, for nearly a decade of steady leadership of the Society and the IDSA Foundation.
A signature moment was the HIV Medicine Association (HIVMA)’s Transformative Leader Award for Demetre Daskalakis, Atlanta, Georgia, USA, who was recognized for 2 decades of work spanning his 'status-neutral' prevention model in New York City, USA, to national leadership roles at the CDC and the White House. His acceptance speech framed a bracing moral call to action. Daskalakis began by reminding the room that “vaccines, antiretroviral therapy, antimicrobials, [and] infectious-disease care, these are all miracles,” born of centuries of science and dedication. An effective response to the current challenges, he argued, rests on “three pillars: science, political will, and co-creation with communities.” Using a striking metaphor, he described today as a kind of “dark age,” urging the field to be the renaissance. When those pillars are strained, the answer is not a retreat but transformative leadership at every level, in clinics and pharmacies, in public health agencies, and in policy. The standing ovation that followed reflected both the moment and the messenger: an equityfirst leader whose recent resignation from the CDC over the politicization of vaccine policy has made his appeal for courage and community even more resonant.
IDSA’s top lifetime honor, the IDSA Alexander Fleming Award for Lifetime Achievement, went
to Cynthia L. Sears from The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA. She was celebrated as a world-renowned physician-scientist, mentor, and leader. The Society noted her career as one that has embodied the virtues the Fleming Award exists to recognize: scientific excellence, mentorship, and service, making her a fitting recipient to open IDWeek’s celebration of the field’s most enduring contributors.
Paige Alexander, CEO of The Carter Center, Atlanta, Georgia, USA, framed infectious diseases within broader issues of institutional trust and global health. The Carter Center was founded by former U.S. President Jimmy Carter and former First Lady Rosalynn Carter on a very simple premise: “Waging peace, fighting disease, and building hope.” From that starting point, the Center has helped 22 countries eliminate at least one neglected tropical disease, distributed more than 1.1 billion doses of medicine, provided sight-restoring surgery to roughly one million people, and
IDWeek’s awards underscored the community’s depth and breadth
monitored more than 125 elections in 48 countries. Most dramatically was the Guinea worm eradication, cutting cases from an estimated 3.5 million a year in 21 countries to just 15 human cases in 2024 through partnerships with local governments and communities. The Carter Center perspective underscored the importance of public trust: “Health security is impossible without public trust; if people don’t believe you, they won’t drink the filtered water, they won’t take the medicine, and they won’t show up for vaccines.”
In the closing minutes of the Plenary, speakers previewed a new collaboration between the Center for Infectious Disease Research and Policy (CIDRAP) and a journal house to publish rapid “Public Health Alerts,”
intended to disseminate vetted outbreak and safety signals at Morbidity and Mortality Weekly Report (MMWR)-like speed.
The Annual Meeting included Meet-theProfessor, career fair, and mentorship sessions, which emphasized the real investment in building a new generation of infectious disease clinicians. The most infectious event, the IDBugBowl, kept everyone on the edge of their seats, with the University of Alabama clinching the win on the final Jeopardy-style question.
The big themes of IDWeek revolved around prevention first. The clinical efficacy of twice-yearly lenacapavir pre-exposure prophylaxis in women, and the very high efficacy in men and gender-diverse people, was highlighted in several discussions.1,2 Additional favorable data on safety and efficacy in youth, pregnant people, and people who use substances were presented. Now that lenacapavir is approved by the FDA, the pressing next step is focusing on accessability and affordability. How will lenacapvair pre-exposure prophylaxis be delivered, paid for, and reach the individuals least likely to stay in care?3
A second theme was respiratory protection. Real-world data on the respiratory syncytial virus vaccination in older adults and patients with immunocompromising conditions reassured clinicians that these vaccines prevent hospitalization and critical illness.4,5 However, the protection wanes over time, especially in immunocompromised people.6

Maternal–infant prevention also got a big boost: a Phase 4, randomized, openlabel study showed that infants have high neutralizing antibody levels whether the mother was vaccinated in pregnancy, the infant received nirsevimab after birth, or both. There were no safety concerns across the board.7
IDWeek emphasized the need for healthcare that finds patients, not the other way around. Inpatient programs that screen for hepatitis C and start direct-acting antivirals at the bedside showed that they can capture people who would otherwise be lost to care after discharge.8 Telehealth-based treatment, as well as point-of-care testing and treatment initiation, were also ways to reach patients, including people who use drugs or those with unstable housing.9
Lastly, on the inpatient infectious diseases practice side, the dalbavancin DOTS trial offered another option for the treatment of complicated Staphylococcus aureus bacteremia with a long-acting agent, sparing patients from peripherally inserted central catheter lines and the logistics of home intravenous therapy.10 New options for S. aureus bacteremia could be on the horizon. In a recent Phase 2a trial, an intravenous bacteriophage cocktail, AP-SA02, with best available therapy showed significantly higher clinical cure rates, earlier resolution of infection, and shorter hospital stays, demonstrating early efficacy signals and a favorable safety profile.11 The PIVOT-PO trial presented the safety and efficacy data of oral tebipenem pivoxil hydrobromide (oral carbapenem) compared with imipenemcilastatin for the treatment of complicated urinary tract infection. Tebipenem showed similar efficacy, including in participants with extended-spectrum beta-lactamaseproducing Enterobacterales.12
References
1. Bekker LG et al.; PURPOSE 1 Study Team. Twice-yearly lenacapavir or daily F/TAF for HIV prevention in cisgender women. N Engl J Med. 2024;391(13):1179-92.
2. Kelley CF et al.; PURPOSE 2 Study Team. Twice-yearly lenacapavir for HIV prevention in men and genderdiverse persons. N Engl J Med. 2025;392(13):1261-76.
3. Fairhead C et al. Generic lenacapavir HIV pre-exposure prophylaxis could be produced for $25 per person per year. Presentation 174. IDWeek, October 1922, 2025.
4. Link-Gelles R et al. Effectiveness of RSV vaccines in older adults in the United States, VISION Network, 2023-2025. Presentation 221. IDWeek, October 1922, 2025.
5. Mayer EF et al. Safety, tolerability, and immunogenicity of the mRNA-1345
RSV vaccine in solid organ transplant recipients aged ≥18 years. Presentation 223. IDWeek, October 19-22, 2025.
6. Hage C et al. Durability of RSV antibodies following RSV vaccination in solid organ transplant recipients. Am J Transplant. 2025;25(8):S98-9.
7. Rostad CA et al. The immunology and safety of maternal RSV vaccination, infant nirsevimab immunization, or both products- interim analysis of a randomized clinical trial. Presentation 225. IDWeek, October 19-22, 2025.
8. McCrary LM et al. large scale implementation of opportunistic HCV treatment during hospitalization in a US tertiary care hospital. Presentation 201. IDWeek, October 19-22, 2025.
9. Di Paola A et al. Highway to health: mobile pharmacy and clinic providing hepatitis C testing and treatment. Presentation 198. IDWeek, October 1922, 2025.
10. Turner NA et al. Dalbavancin for treatment of Staphylococcus aureus bacteremia: the DOTS randomized clinical trial. JAMA. 2025;DOI:10.1001/ jama.2025.12543.
11. Miller LG et al. A phase 2a randomized, double-blind, controlled trial of the efficacy and safety of an intravenous (IV) bacteriophage cocktail (APSA02) vs. placebo in combination with best available antibiotic therapy (BAT) in patients with complicated Staphylococcus aureus bacteremia. Presentation 549. IDWeek, October 1922, 2025.
12. Hong DK et al. Oral tebipenem pivoxil hydrobromide versus intravenous imipenem-cilastatin in patients with complicated urinary tract infections or acute pyelonephritis: efficacy and safety results from the phase 3 PIVOTPO study. Presentation 173. IDWeek, October 19-22, 2025.
Author: *Sara Hockney
1. Northwestern University, Feinberg School of Medicine, Chicago, Illinois, USA
*Correspondence to sara.hockney@northwestern.edu
Disclosure: The author has declared no conflicts of interest.
Keywords: Cabotegravir (CAB), global health, HIV, HIV prevention, implementation science, lenacapavir (LEN), long-acting (LA) injectables, pre-exposure prophylaxis (PrEP).
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:27-31. https://doi.org/10.33590/microbiolinfectdisam/JCTG4390
THIS YEAR'S IDWeek in Atlanta, Georgia, USA, highlighted a turning point in HIV prevention, as researchers and clinicians gathered to discuss advances redefining the field. The focus has shifted decisively toward long-acting, simplified, and patient-centered prevention strategies. The data presented underscored both the remarkable efficacy of new agents such as cabotegravir (CAB) and lenacapavir (LEN), and the growing emphasis on equitable implementation, real-world monitoring, and access. From twice-yearly injectables to next-generation oral and biologic approaches, the landscape of pre-exposure prophylaxis (PrEP) is rapidly evolving, promising improved adherence, convenience, and meaningful narrowing of persistent prevention gaps.
CABOTEGRAVIR FOR PREEXPOSURE PROPHYLAXIS: FROM TRIALS TO IMPLEMENTATION
Long-acting injectable CAB (CAB-LA), administered every 2 months, continues to demonstrate robust efficacy. In the US PrEPFACTS study, only three participants (0.2%) had evidence of seroconversion over a median follow-up of 325 days, although HIV testing only occurred in about 60% of injections, highlighting ongoing gaps in monitoring.1
Safety data for CAB-LA during pregnancy are increasingly reassuring, addressing a critical knowledge gap. The open-label extension of HPTN 084 monitored cisgender
women who became pregnant while receiving CAB-LA and found pregnancy and infant outcomes comparable to population-level expectations.2,3 Maternal adverse effects, specifically gestational hypertension, were more frequent in those receiving CAB-LA, although serious complications were rare.3 Pharmacokinetic analyses demonstrated that CAB concentrations remain above protective thresholds across all trimesters, suggesting that no dose adjustments are necessary.3 While ongoing surveillance remains essential, these data provide clinicians and patients with evidence to support informed decisionmaking during pregnancy, a population historically underrepresented in clinical trials.
Carina Marquez, University of California, San Francisco, USA, reviewed the landmark PURPOSE 1 and PURPOSE 2 trials. PURPOSE 1, conducted among young women in subSaharan Africa, reported no HIV infections in the LEN arm, demonstrating 100% efficacy, although two seroconversions occurred in extended follow-up.4 PURPOSE 2, which included a broader, gender-diverse population, demonstrated a 96% reduction in HIV incidence. 4 These results led to FDA approval of LEN for PrEP in June 2025, offering the first twice-yearly injectable option and a potential solution to adherencerelated challenges.
Practical considerations for LEN include injection-site reactions and the need for an oral loading dose to achieve immediate protection. Additionally, LEN is both a substrate for and moderate inhibitor of CYP3A4, creating potential drug–drug interactions. Strong inducers, such as rifampin, require dose adjustments, but
dose adjustments are not needed for oral contraceptives or gender-affirming hormone therapy.4 Encouraging data on reproductive safety were also presented. In a nested pregnancy substudy within PURPOSE 1, over 480 participants became pregnant while receiving LEN. Rates of miscarriage, stillbirth, and preterm delivery were comparable to background population rates.2,4 LEN concentrations remained consistent during pregnancy, and minimal transfer was observed through breast milk.4
While new LA agents drew considerable attention, IDWeek 2025 also highlighted the broader systems-level factors shaping HIV prevention success. Centers for Disease Control and Prevention (CDC) data demonstrated that, between 2012–2022, mean state-level PrEP coverage increased from 0.6% to 26.3%, accompanied by a decline in HIV diagnosis rates from 13.0 to 10.6 per 100,000 people.3 States in the highest quintile of PrEP coverage experienced

the steepest declines, confirming an inverse dose–response relationship between PrEP uptake and new infections.
Despite overall progress, wide regional and demographic disparities persist. Ofole Mgbako, NYC Health + Hospitals, New York, USA, reviewed the PrEP-to-need ratio, the number of PrEP prescriptions relative to HIV incidence, as a marker of equitable access.5 He noted substantial racial and ethnic inequalities across the USA. Josh Havens, University of Nebraska, Lincoln, USA, reported that only about 36% of individuals eligible for PrEP currently receive it.6 Coverage remains highest among White individuals at 94%, dropping to 24% for Hispanic/Latino persons, and only 13% for Black individuals.6
financial and insurance-related barriers impede access. A 2024 national Walgreens survey found that 19% of individuals did not fill their first PrEP prescription within 14 days, primarily due to cost and coverage considerations.9 These findings underscore that affordability and streamlined insurance authorization remain central to achieving equitable HIV prevention outcomes.
Economic barriers remain a major deterrent to PrEP initiation and further compound existing inequities in access
There is also inequitable access among women, who make up 19% of new HIV diagnoses but only 8% of PrEP users.5 Data from the STAR cohort, a longitudinal study of reproductive-age women without HIV in the Southern USA, showed that among 362 eligible women, only 9.9% had ever used PrEP.7 Barriers included cost, provider access, and inconsistent use, highlighting that clinical efficacy alone is insufficient without attention to access, equity, and patient-centered delivery.
Economic barriers remain a major deterrent to PrEP initiation and further compound existing inequities in access. Manufacturing analyses estimate that LEN could be produced for as little as 25 USD per person per year, yet USA list prices currently exceed 28,000 USD annually.⁸ Even for older oral PrEP regimens,
While equitable access and affordability remain central to PrEP uptake, data presented at IDWeek 2025 emphasized that the real-world success of LA prevention depends on effective clinical integration. Implementation studies demonstrated that embedding PrEP within existing healthcare systems, through primary care, sexual health, pharmacy, and community-based settings, reduces burden on patients and providers while improving retention.5,6 Engaging nonphysician healthcare workers, including nurses, pharmacists, and peer navigators, as well as leveraging mobile health clinics and telehealth platforms, further enhances engagement, particularly among marginalized populations.6
Real-world examples illustrate these dynamics: telehealth-based initiation of emtricitabine/tenofovir alafenamide fumarate PrEP at Vivent Health clinics achieved high satisfaction and 3-month followup, while the EquiPrEP study at Bellevue Hospital in New York, USA, found that over 83% of participants, including Black/ Latine cisgender men who have sex with men, Black/Latine cisgender women, and transgender/nonbinary persons, were fully adherent to LA injectable PrEP, continued on LA injectable PrEP, or switched to oral PrEP over 6 months.5,10,11 These findings highlight the value of community-based partnerships, flexible delivery models, and strategies that address structural and patient-level barriers to sustain adherence and engagement.
Monitoring strategies remain a topic of active discussion. The 2021 CDC guidelines recommend both HIV antigen/antibody and RNA testing at PrEP initiation and follow-up to minimize the risk of starting or continuing PrEP during acute infection. Updated data presented by Collen Kelley, Emory University, Atlanta, Georgia, USA, using the HealthVerity database (2018–2023), challenge the incremental value of this dual approach. Following the 2021 guideline update, RNA testing among oral PrEP users increased more than sevenfold, yet positivity rates fell from 1.39% to 0.22%, and the positive predictive value dropped from 100% to 67%, translating to roughly 9,000 RNA tests required to identify one additional early infection compared with antigen/antibody testing alone.3 Reflecting these findings, International Antiviral Society-USA (IASUSA) guidelines now omit follow-up HIV RNA monitoring for CAB-LA based on HPTN 083 data, and LEN protocols similarly do not require HIV RNA monitoring.3 While CDC

guidance remains unchanged, the research suggests that routine RNA testing for asymptomatic individuals on LA PrEP may offer limited clinical benefit relative to cost and resource use.
Building on these clinical integration and monitoring strategies, emerging LA and nextgeneration PrEP modalities offer additional opportunities to expand coverage and simplify HIV prevention. Results of the Phase I clinical trial of once-yearly intramuscular LEN demonstrated higher trough levels than were seen in PURPOSE 1 and PURPOSE 2, and an additional Phase III trial is underway looking at a once-a-year lower dose with concomitant oral load.3 Ultra CAB-LA is also under investigation as an intramuscular or subcutaneous injection every 4 months.4
Beyond injectables, IDWeek 2025 also spotlighted novel modalities poised to expand PrEP’s reach. The investigational oral agent MK-8527, a next-generation nucleoside reverse transcriptase translocation inhibitor, showed promise as a once-monthly oral PrEP option in earlyphase trials.3,4 If validated in later studies, it may offer a valuable alternative for those who prefer pills to injections while easing adherence demands compared with daily regimens.
Researchers also presented encouraging data on broadly neutralizing antibodies as prevention tools. In a Phase I study, newborns exposed to HIV received VRC07523LS, a long-acting monoclonal antibody, in addition to standard antiretroviral prophylaxis. The intervention was safe, well tolerated, and achieved sustained serum concentrations.3 Combinations of broadly neutralizing antibodies are also under investigation as an intravenous infusion given every 6 months.
Despite advancements in LA HIV prevention, barriers continue to limit global impact. In many low- and middle-income countries, HIV prevention remains highly dependent on external funding streams, including PEPFAR and the Global Fund to Fight AIDS, TB, and Malaria, leaving programs vulnerable to USA policy changes. In Malawi, abrupt reduction in USA support in early 2025 led to the suspension of community-based testing and prevention services, while treatment programs continued, demonstrating the vulnerability of prevention infrastructure and the risk of reversing epidemic control gains.5 If such cuts continue, modeling data predict a 50% increase in new HIV infections in Africa over the next 5 years, equating to 4.4–10.8 million additional new infections.5
Looking ahead, the introduction of LEN at a projected cost of 40 USD per patient per year in 120 high-incidence, resource-limited
References
1. Metzner A et al. Human immunodeficiency virus (HIV) testing and evidence of HIV amount real-world long-acting pre-exposure prophylaxis (PrEP) users in a United States claims database: results from the PrEPFACTS study. Presentation 574. IDWeek, October 19-22, 2025.
2. Rana A et al. Afternoon delight: challenging HIV and STI coinfection cases. Presentation 78. IDWeek, October 19-22, 2025.
3. Kelley C. What’s hot in HIV clinical sciences. Presentation 7. IDWeek, October 19-22, 2025.
4. Marquez C. State of the ART HIV prevention. Update in therapeutics. Presentation 118. IDWeek, October 19-22, 2025.
countries starting in 2027 represents a potential step forward.5 PEPFAR and Global Fund are likely to prioritize LEN implantation for an estimated two million people by 2028.5 However, this focused investment may inadvertently limit patient choice, as other PrEP formulations may become less accessible in these regions.
LA HIV prevention, including CAB and LEN, is reshaping PrEP delivery by improving adherence and offering flexible options for diverse populations. Success depends not only on clinical efficacy, but also on equitable access, real-world integration, and sustainable implementation in the USA and globally. Bridging scientific innovation with structural and social considerations is essential to closing prevention gaps and reducing new infections worldwide.
5. Mgbako O. State of the ART HIV prevention. The scope and reach of HIV prevention access: the road ahead. Presentation 118. IDWeek, October 19-22, 2025.
6. Havens J. State of the ART HIV prevention. Bridging the gap with innovation: implementation strategies for HIV prevention. Presentation 118. IDWeek, October 19-22, 2025.
7. Carr E et al. PrEP Use among women of reproductive age enrolled in the study of treatment and reproductive outcomes. Poster P-301. IDWeek, October 19-22, 2025.
8. Fortunak J et al. Generic lenacapavir hiv pre-exposure prophylaxis could be produced for $25 per person per year. Presentation 174. IDWeek, October 19-22, 2025.
9. Sullivan P et al. Barriers to oral HIV preexposure prophylaxis (PrEP) perceived by those receiving an initial prescription: US survey analysis. Poster P-326. IDWeek, October 19-22, 2025.
10. Firnhaber C et al. Telehealth as a modality to improve the uptake of PrEP services in Black and Latino MSM “ePrEP”. Poster P-296. IDWeek, October 19-22, 2025.
11. Mgbako O et al. Examining preliminary adherence to long-acting injectable pre-exposure prophylaxis (LAI-PrEP) among racial, sexual, and gender minority populations at NYC Health + Hospitals/Bellevue: the EquiPrEP Study. Poster P-323. IDWeek, October 19-22, 2025.

This collection of abstracts showcases developments across diverse infectious diseases, addressing real-world challenges relevant to modern clinical practice. Topics include optimizing HIV virologic suppression; sexually transmitted infection and tuberculosis screening in incarcerated populations; evaluating diagnostic tools; and stewardship interventions in pneumonia and bloodstream infections.
Authors: Hadeel Fouad,1 Melissa E. Badowski,1,2 Joy Lee,1 Jennifer Flores,1 Jane Park,3 Brian Drummond,2 Mahesh Patel,2,3 Scott Borgetti,2,3 Drew Halbur,4 *Emily N. Drwiega,1,2
1. University of Illinois Retzky College of Pharmacy, Chicago, USA
2. University of Illinois Hospital & Health Sciences System Chicago, USA
3. University of Illinois Chicago, USA
4. Walgreens Pharmacy, Chicago, Ilinois, USA
*Correspondence to edrwiega@uic.edu
Disclosure: The authors have declared no conflicts of interest.
Keywords: Antiretroviral therapy, correctional medicine, HIV, reincarceration, telemedicine.
Citation: Microbiol Infect Dis AMJ. 2025;3[1]: 33-34. https://doi.org/10.33590/microbiolinfectdisam/LCMO3544
The University of Illinois Hospital and Health Sciences Center (UIH), Chicago, USA, multidisciplinary telemedicine clinic provides HIV care to justice-involved individuals in the Illinois Department of Corrections (IDOC).1 Post-release HIV care remains challenging, with virologic suppression (VS) dropping from 73% at release to 49.7% at reincarceration into IDOC, based on previous data.2 To improve continuity of care, wrap-around services were expanded from follow-up care at UIH to medical insurance assistance, case management, employment/housing support, and statewide follow-up care.3
This Institutional Review Board (IRB)approved, retrospective cohort study occurred from January 1, 2021–August 31, 2024 and analyzed demographic, clinical,
and social determinants of health in people with HIV aged 18 years or older, who were in custody in IDOC, released, and reincarcerated during the study period. The primary outcome was a change in the number of patients with complete VS at the time of release as compared to reincarceration in those who were virologically suppressed at release.4 Secondary outcomes included change in immunologic function and identification of factors influencing loss of VS upon reincarceration.
Of 393 patients released during the study period, 95 were reincarcerated, 82 were included, and 75 had VS at the time of release. Of the 75 who achieved VS at release, 62 maintained VS (Group 1) at reincarceration, while 13 experienced loss of VS (Group 2; p=0.0001). Median cluster of differentiation (CD)4 count of those who met inclusion criteria declined significantly from release to reincarceration (p=0.0032), but the change in CD4% was not significant (p=0.0973; Table 1).
No difference was found between Group 1 and 2 in scheduling a statewide followup visit (p=0.0813), following up at UIH clinic at least once (p=1.00), or having AIDS Drug Assistance Program (ADAP) coverage (p=0.7552). Loss of VS was significantly associated with patient-reported housing instability (p=0.0046), lack of access to care (p=0.0056), living outside of Chicago (p=0.0318), and transferring from a jail in a rural setting (p=0.0054).
CD4 count (cells/mm3; IQR)
Number of patients with CD4 count <200 cells/mm3 (%) 4/82 (4.9%) 4/82 (4.9%)
*Patients who were not suppressed at release: four patients were released before the lab results could be updated, two patients preferred no pharmacotherapy, and one patient was not started on medication because the team decided it would be more effective to initiate treatment after release to ensure better follow-up and continuity of care.
CD: cluster of differentiation; IQR: interquartile range; NS: not significant; VL: viral load.
As the role of the IDOC telemedicine team expanded, the proportion of individuals who maintained VS upon reincarceration increased from 49.7% in 2014 to 82.7% in 2024. While interventions show progress, more targeted efforts are needed to address housing, care access, and non-Chicago residency.
1. Fouad H et al. Evaluation of human immunodeficiency virus (HIV) virologic suppression among reincarcerated individuals within in the illinois department of corrections. Poster. IDWeek, October 19–22, 2025.
2. Centers for Disease Control and Prevention (CDC). HIV Diagnoses, deaths, and prevalence: 2025 update. 2025. Available at: https://www.cdc.gov/hiv-data/ nhss/hiv-diagnoses-deaths-and-prevalence-2025. html. Last accessed: 19 August 2025.
3. Widra E. New data on HIV in prisons during the COVID-19 pandemic underscore links between HIV and incarceration. 2023. Available at: https://www. prisonpolicy.org/blog/2023/06/01/hiv_in_prisons/. Last accessed: 19 August 2025.
4. Badowski ME, Patel M. Evaluation of immunologic and virologic function in reincarcerated patients living with HIV or AIDS. J Correct Health Care. 2022;28(3):203-6.
Authors: *Sara Hockney,1 Dana Mueller,2 Alex Phillbrick,1 Jenna Berlet,1 Shannon Galvin1
1. Division of Infectious Diseases, Department of Medicine, Northwestern University, Chicago, Illinois, USA
2. Southwest Infectious Disease & Internal Medicine, Palos Heights, Illinois, USA *Correspondence to sara.hockney@northwestern.edu
Disclosure: Galvin has received research funding from GSK, unrelated to the current article. The other authors have declared no conflicts of interest.
Keywords: Anti-retroviral therapy (ART), cabotegravir-rilpivirine (CAB/RPV), HIV, longacting (LA) injectables.
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:3536. https://doi.org/10.33590/microbiolinfectdisam/ WNZA9304
Long-acting (LA) injectable cabotegravirrilpivirine (CAB/RPV) is a novel antiretroviral therapy (ART) option for virologically suppressed persons with HIV (PWH) who have no prior treatment failure and no known or suspected resistance to either agent.1 However, in practice, patients often initiate LA CAB/RPV with incomplete or unknown treatment and resistance histories. This study aimed to characterize reasons for LA CAB/RPV therapy discontinuation in clinical practice and to describe a subset of cases in which virologic failure occurred.
The authors retrospectively reviewed all patients seen in an infectious diseases clinic between January 1, 2022–April 17, 2025 who were on LA CAB/RPV for HIV treatment.2 Virologic failure was defined as two or more consecutive viral load measurements ≥200 copies/mL. Statistics were performed in R version 4.4.2.
During the study period, 201 PWH were treated with LA CAB/RPV for an average of 472 days (range: 0–1,241). Those on LA CAB/RPV were predominantly male (85.6%) with a mean age of 47 years. Therapy was discontinued in 41 (20%) patients. Reasons for discontinuation included transfer of care (n=11; 27%), insurance (n=8; 20%), adherence (n=7; 17%), patient preference (n=7; 17%), virologic failure (n=4; 10%), intolerance (n=3; 7%), and death (n=1; 2%). Combined genotype/phenotype testing was done at the time of failure in the four patients who discontinued therapy due to virologic failure (Table 1). Two had RPV resistance, one had integrase resistance, and one had both RPV resistance and intermediate CAB resistance. One additional patient experienced transient virologic failure with a maximum viral load of 1,230 copies/mL. Resistance testing was negative, and virological suppression was subsequently achieved with no change in therapy. There was no difference in BMI between patients who experienced virologic failure and those who did not (32.06 kg/m2 versus 28.92 kg/m2; p=0.11).
Table 1: Clinical characteristics, resistance profiles, and outcomes of patients with virologic failure on long-acting injectable cabotegravir-rilpivirine.
42, M Genotype negative for relevant mutations (4 years prior)
60, M None on file
43, M Phenotype with resistance to DTG, EVG, RAL (1 year prior)
47, M
None on file
Viral blips for 20 m, then ↑ to 2,350 at 21 m K101P
First VL at 5 m 192, then ↑ to 13,100 at 9 m
First VL at 3 m 17,700
High-level RPV resistance
Y181C Intermediate RPV resistance
Not assessed
First VL at 6 m 386,000 E138A M230L
61, M None on file VL <20 for 4 m then ↑ to 1,230 at 5 m
None
Resistance to DTG, EVG, RAL, BIC
High-level RPV resistance; partial sensitivity to CAB
Sensitive to RPV, DTG, EVG, RAL, BIC
2. Hockney S et al. Discontinuation patterns and virologic failure among persons with HIV receiving long-acting injectable cabotegravir-rilpivirine antiretroviral therapy. Poster P-384. IDWeek, October 19-22, 2025. Patient (age, sex)
Resumed prior regimen of FTC/TAF, DTG + DOR, and resuppressed
Resumed prior regimen of ATV/r + ABC/3TC, and resuppressed
Resumed prior regimen of DRV/ COBI/FTC/TAF, and re-suppressed
Resumed prior regimen of DRV/ COBI/FTC/TAF, and re-suppressed
Continued LA CAB/RPV, and resuppressed by 7 m
ABC/3TC: abacavir/lamivudine; ATV/r: atazanavir/ritonavir; BIC: bictegravir; CAB: cabotegravir; DOR: doravirine; DRV/COBI: darunavir/cobicistat; DTG: dolutegravir; EVG: elvitegravir; FTC/TAF: emtricitabine/tenofovir alafenamide; LA: long-acting; m: months; M: male; RAL: raltegravir; RPV: rilpivirine; VL: viral load.
In PWH on LA CAB/RPV, discontinuation occurred primarily due to transfer of care and insurance barriers. Virologic failure was rare and was associated with underlying resistance but not BMI. These findings highlight the tolerability of LA CAB/RPV in clinical practice and the need to address access issues to optimize patient outcomes.2
References
1. FDA. Cabenuva. 2021. Available at: https:// www.accessdata.fda.gov/drugsatfda_docs/ label/2021/212888s000lbl.pdf. Last accessed: 27 October 2025.
Authors: Danny Schreiber,¹ Emily N. Drwiega,¹ Miguel Perez,1 Rita Uda,1 Mahesh Patel,2 Scott Borgetti,2 *Melissa Badowski¹
1. Department of Pharmacy Practice, Retzky College of Pharmacy, University of Illinois Chicago, USA
2. Division of Infectious Diseases, Department of Medicine, College of Medicine, University of Illinois Chicago, USA
*Correspondence to badowski@uic.edu
Disclosure: Borgetti has received grants or contracts from GSK for an RSV vaccine study. The other authors have declared no conflicts of interest.
Keywords: Chlamydia, co-infection, corrections, gonorrhea, HIV/AIDS, retrovirus, sexually transmitted infection (STI), syphilis.
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:3738. https://doi.org/10.33590/microbiolinfectdisam/ RRVF1763
Sexually transmitted infections (STI) have a disproportionately high prevalence in individuals in custody and persons with HIV (PWH). However, limited data exist for the rates of infection in individuals who are in custody, particularly PWH, leading to a potential gap in timely and appropriate recognition and treatment of STIs. In the Illinois Department of Corrections (IDOC), persons in custody are screened at initial intake and those with HIV are seen by a multidisciplinary care team via telemedicine to manage HIV care and related STIs.1,2
Electronic medical records of PWH receiving care via IDOC telemedicine in conjunction with the University of Illinois Hospital and
Health Sciences System, Chicago, USA, were reviewed from January 1st, 2021–June 30th, 2024. The primary objective was to determine the frequency of screening and positivity for gonorrhea, chlamydia, and syphilis. HIV viral load and other known STI risk factors were also collected to assess predictors of STI positivity.
The majority of PWH in IDOC were Black, cisgender males. Of 241 patients with HIV in IDOC, a total of 226 (94%), 98 (41%), and 97 (40%) patients were screened for syphilis, gonorrhea, and chlamydia, respectively (Table 1). Of those screened at intake, 218/226 (96%), 26/98 (27%), and 26/97 (27%) were screened for syphilis, gonorrhea, and chlamydia, respectively. Following linkage to care with the multidisciplinary telemedicine team, more patients were screened for gonorrhea (70/98; 88%) and chlamydia (71/97; 73%) compared to intake. Sixty-five out of 226 (29%), 3/98 (3%), and 2/97 (2%) patients were positive for syphilis, gonorrhea, and chlamydia, respectively, with 41/65 (63%) representing new syphilis diagnoses. STI positivity was associated with women who were transgender (p=0.02), men who have sex with men (p<0.001), and a history of STI (within 12 months prior to intake; p<0.001). Linkage to care with the University of Illinois Health HIV telemedicine team had significantly more gonorrhea and chlamydia screenings than screening upon intake (p<0.001).
The majority of patients were screened for syphilis during their time in custody and approximately one in five were newly diagnosed. Gonorrhea and chlamydia were infrequently screened with low rates of positivity.3
Table 1: Sexually transmitted infection screening.
Screened for STI (composite), n (%)
229 (95)
Screened for NG, n (%) 98 (41)
At intake 28 (12)
Post-linkage to care with UIH Telemedicine Team 70 (88)
Screened for CT, n (%) 97 (40)
At intake 26 (27)
Post-linkage to care with UIH Telemedicine Team 71 (73)
Screened for syphilis, n (%) 226 (94)
At intake 218 (96)
Post-linkage to care with UIH Telemedicine Team 16 (7)
p<0.001
p<0.001
p<0.001
CT: Chlamydia trachomatis; NG: Neisseria gonorrhoeae; STI: sexually transmitted infection; UIH: University of Illinois Hospital and Health Sciences System.
Routine screening for STIs for PWH by multidisciplinary care teams has the potential to identify a high proportion of STIs, with key patient demographics and past medical history serving as potential predictors of STI positivity.
References
1. Schreiber D et al. Screening and incidence of sexually transmitted infections (STI) among persons living with HIV (PWH) in the Illinois Department of Corrections (IDOC). Poster P-229. IDWeek, October 19-22, 2025.
2. Cloud DH et al. Public health and prisons: priorities in the age of mass incarceration. Annu Rev Public Health. 2023;44:407-28.
3. Dang CM et al. Paired testing of sexually transmitted infections with urine pregnancy tests in incarcerated women. Sex Transm Dis. 2021;48(8S):S20-5.
Authors: Luke Stickler,1 Daniel McKelvey,1 Tommy Windt,1 Mahesh Pate,2 Scott Borgetti,2 Emily N. Drwiega,1 *Melissa E. Badowski1
1. Retzky College of Pharmacy, University of Illinois Chicago, USA
2. College of Medicine, University of Illinois Chicago, USA
*Correspondence to badowski@uic.edu
Disclaimer: Borgetti has received a grant from GSK for an RSV vaccine trial. The other authors have declared no conflicts of interest.
Keywords: Department of corrections, drug–drug interactions (DDI), HIV, latent tuberculosis infection, Mycobacterium tuberculosis, screening, tuberculosis (TB).
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:3940. https://doi.org/10.33590/microbiolinfectdisam/ IHCM1779
Tuberculosis (TB) screening is crucial for people in custody, as people with HIV (PWH) in custody face a compounded risk. This study investigated TB screening incidence, detection, and treatment practices of PWH in custody within the Illinois Department of Corrections (IDOC).1
The authors conducted a retrospective chart review of PWH serviced by the University of Illinois Health (UIH) IDOC telemedicine HIV clinic. Adult PWHs under care of UIH’s HIV telemedicine team during custody from January 2021–October 2024 were included in the study. If an individual was reincarcerated during the study period, each unique encounter was included. The primary objective was the incidence of PWH screened for TB in IDOC. Secondary objectives were incidence of latent or active TB, appropriateness of TB or latent TB infection treatment, drug–drug interactions (DDI), and viral and immunologic function at intake versus release or completion of TB treatment, whichever occurred first.
Of the participants, 91.5% were male, 76.4% were Black, and the average age was 37.5 years. TB screening occurred in 96.5% (249) of PWH in custody, with 95.6% (238/249) being negative, 2.4% (6/249) indeterminate, 2% (5/249) positive, and 0% with active TB (Figure 1). Six required latent TB treatment
(only two were guideline-appropriate), 83.3% (5/6) of whom possessed DDIs, and 100% of DDIs were identified and mitigated by the HIV telemedicine team. At release, all PWH treated for latent TB infection achieved CD4 counts >200 cells/mm³ and viral suppression (<200 copies/mL).
The study found high TB screening incidence and low latent TB rates with no active TB. The UIH telemedicine team played a major role in identifying and mitigating TB and HIV DDIs, and recommending TB screening post-intake, which may have otherwise been missed. Favorable HIV outcomes were maintained during custody while receiving latent TB treatment. A need for TB and HIV treatment education was identified for IDOC providers.
Reference
1. Stickler L et al. Incidence of Mycobacterium tuberculosis screening and detection in people living with human immunodeficiency virus in custody within the Illinois Department of Corrections. Poster P-1412. IDWeek, October 19-22, 2025.
Authors: Curtis Sera,1 Niki Arab,2,3 Brian Kim,2,3 Hera Maryam,4 *Arthur Jeng1,3,4
1. Department of Medicine, Olive View-UCLA Medical Center, Sylmar, California, USA
2. Department of Pharmacy, Olive View-UCLA Medical Center, Sylmar, California, USA
3. Department of Medicine, Division of Infectious Diseases, Olive View-UCLA Medical Center, Sylmar, California, USA
4. Department of Medicine, Division of Infectious Diseases, UCLA David Geffen School of Medicine, Los Angeles, California, USA
*Correspondence to ajeng@dhs.lacounty.gov
Disclosure: The authors have declared no conflicts of interest.
Keywords: Antibiotic de-escalation, Antimicrobial Stewardship Program (ASP), Gram stain (GS), pneumonia, respiratory culture, stewardship.
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:4143. https://doi.org/10.33590/microbiolinfectdisam/ YIOW6925
Gram stain (GS) and BioFire® FilmArray® Pneumonia Panel (PN; Biomérieux, Marcyl’Étoile, France) are rapid methods of detecting bacterial pneumonia pathogens. PN is a highly sensitive and specific tool that rapidly identifies numerous organisms and is validated for use on all types of respiratory specimens.1 While not widely used for this purpose, recent studies have suggested that GS may be used to guide antibiotic de-escalation for pneumonia in which Pseudomonas aeruginosa (PsA) and methicillin-resistant Staphylococcus aureus (MRSA) are suspected.2 This study seeks to evaluate the sensitivity of GS versus PN and GS versus bronchoalveolar lavage (BAL) culture (cx) to determine the utility of GS for antibiotic de-escalation.
This is a single center retrospective study from 1/2021–1/2024 on adults ≥18 years of age admitted for pneumonia. Respiratory sample GS sensitivity for detecting MRSA and PsA was measured using PN or BAL cx on the same specimen as reference standards. PN results were pooled for all respiratory specimen types (e.g., expectorated sputum, tracheal aspirate, and BAL) due to a small number of BAL specimens with PN done on them. Wilson 95% CIs were calculated for each measured test characteristic.
A total of 57 cases in the BAL cx group (mean age: 57 years) and 149 cases in the PN group (mean age: 58 years) were reviewed. In the BAL cx group, there were 11 cases of PsA, four cases of MRSA, and eight of any type of S. aureus as identified in the BAL cx. Meanwhile, PN identified 32 cases of PsA, 22 cases of MRSA, and 47 cases of any type of S. aureus.
The sensitivity of GS with BAL cx as the reference standard was 36% for PsA (95% CI: 15–65%); Figure 1A). The sensitivity for MRSA was comparable at 40% (95% CI: 12–77%), with a general S. aureus sensitivity of 44% (95% CI: 19–73%). The sensitivity of GS using PN as the reference was 78% for PsA (95% CI: 61–89%), 82% for MRSA (95% CI: 61–93%), and 60% for S. aureus overall (95% CI: 45–72%; Figure 1B). Subgroup analysis of the PN specimens that were tracheal aspirates or from BAL showed similar sensitivities to the pooled PN results (Figure 1C). In the subgroup, GS sensitivity was 85% for PsA (n=13; 95% CI: 58–96%), 92% for
Figure 1: Gram stain sensitivity across organisms using different reference standards.
A GS sensitivity with BAL cx as reference
B GS sensitivity with PN as reference
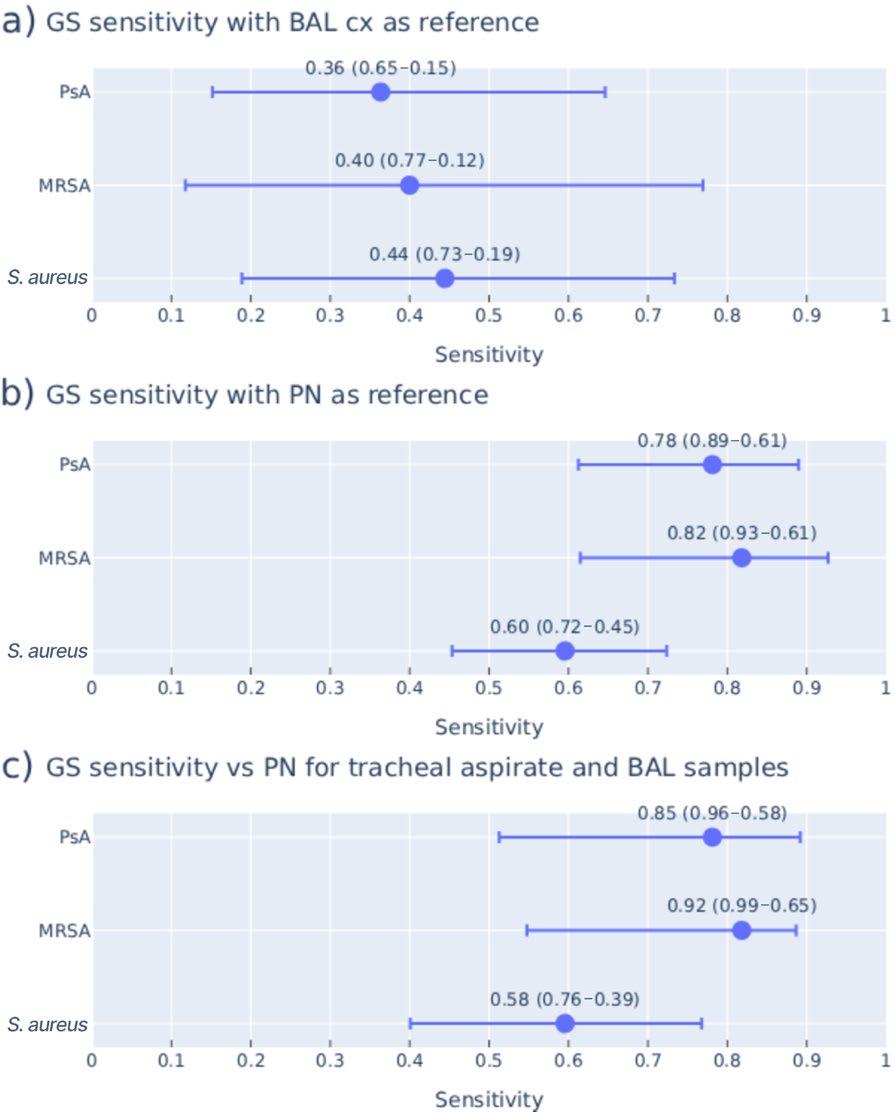
C GS sensitivity vs PN for tracheal aspirate and BAL samples
GS sensitivity using A) BAL cx and B) PN as the reference standards. C) GS sensitivity using PN as the reference only for the subgroup of samples that were tracheal aspirate or also had BAL cx done.
BAL: bronchoalveolar lavage; cx: culture; GS: Gram stain; MRSA: methicillin-resistant Staphylococcus aureus; PN: BioFire® FilmArray® Pneumonia Panel (Biomérieux, Marcy-l’Étoile, France); PsA: Pseudomonas aeruginosa; vs: versus.
MRSA (n=12; 95% CI: 65–99%), and 58% for S. aureus overall (n=24; 95% CI: 39–76%).
Performance of PN was compared to formal culture from any source. For PsA (n=19), sensitivity was 100% (95% CI: 83–100%) with a specificity of 94% (95% CI: 74–99%). For MRSA (n=8), sensitivity was 100% (95% CI: 68–100%) with a specificity of 96% (95% CI: 62–99%).
GS had poor sensitivity for PsA, MRSA, and S. aureus in general compared to BAL cx. GS appeared to perform better compared to PN than to BAL cx, a difference likely due to false
positives from normal respiratory flora found in the non-BAL specimens. However, even with the higher sensitivity in the PN group, GS would still miss at least 20% of true PsA and MRSA infections, which raises concerns about use in critically ill patients.
References
1. Yoshimura J et al. Effect of gram stain–guided initial antibiotic therapy on clinical response in patients with ventilator-associated pneumonia: the GRACEVAP randomized clinical trial. JAMA Netw Open. 2022;5(4):e226136.
2. Murphy CN et al. Multicenter evaluation of the BioFire FilmArray pneumonia/pneumonia plus panel for detection and quantification of agents of lower respiratory tract infection. J Clin Microbiol. 2020;58(7):e00128-20.
Authors: *Jovan Borjan,1 Micah M. Bhatti,2 Nancy N. Vuong,1 Guy Handley,3,4 Amy Spallone3,4
1. Division of Pharmacy, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA
2. Department of Laboratory Medicine, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA
3. Department of Infectious Diseases, Infection Control and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA
4. Department of Infection Control, Chief Quality Office, The University of Texas MD Anderson Cancer, Houston, Texas, USA
*Correspondence to JBorjan@mdanderson.org
Disclosure: Bhatti has received payments from Accelerate Diagnostics and bioMérieux Inc. for lectures, presentations, or related educational activities. The other authors have declared no conflicts of interest.
Acknowledgements: The authors would like to thank MD Anderson Cancer Center Infectious Diseases faculty, fellows, Advanced Practice Providers, and PharmDs for championing conservation efforts; institutional providers, care teams, and nurses for embracing judicious use of blood culture orders during the shortage period; and the Finance and Analytics team for ambulatory cost data.
Keywords: Antimicrobial stewardship, bacteremia, blood culture shortage, blood culture stewardship, diagnostic stewardship, immunocompromised stewardship.
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:4446. https://doi.org/10.33590/microbiolinfectdisam/ LFJI5993
A critical global supply chain shortage resulted in the CDC recommending hospitals establish conservation plans in late July 2024 for Becton Dickson (BD) BACTEC blood culture bottles (BD, Franklin Lakes, New Jersey,
USA).1 Data on the clinical results from these conservation efforts are lacking, particularly in high-risk immunocompromised patients.
The objective of this study was to describe the outcomes of blood culture (BCx) stewardship interventions aimed at conserving BCxs during the shortage while preserving patient care and safety.
Each BCx accounted for two blood culture bottles. Utilization and positivity rates were determined pre-intervention (1/1/2024 to 7/31/2024), and a goal reduction in daily use was established. Conservation efforts to reduce daily utilization of BCx included: removing recurring and as-needed BCx order options, discontinuing surveillance BCx, electronic health record alert for ordering BCx within 48 hours of a prior order or in the outpatient setting, education on highyield versus low-yield clinical scenarios for BCx, and modifications to acute cancer care center sepsis alerts. A BCx utilization rate post-intervention (8/1/2024 to 12/31/2024) was tracked through a developed electronic health records dashboard. BCxs drawn, BCX positivity rates, and organism distribution were assessed pre- and post-intervention. BCxs drawn were standardized by days present (the number of patients present at any time on a given day in each patient care location) to account for patient volume over time. The resulting values are represented as BCxs per 1,000 days present. Skin flora contaminants were defined as BCx growing any coagulasenegative Staphylococci (non-lugdunensis), Bacillus (non-cereus) spp., Corynebacterium (non-jeikeium) spp., and Micrococcus spp. without the presence of concomitant Gramnegative or pathogenic Gram-positive
organisms. Descriptive statistics were calculated using mean values and compared using the Wilcoxon rank-sum test.
During the intervention period, diagnostic stewardship efforts decreased BCxs drawn from 224 to 99 BCx per 1,000 days present (p=0.001) while maintaining overall BCx positivity rate at 12.1% compared to 11.3% preintervention (p=0.183). Skin flora recovery rate decreased from 2.00 to 1.03 contaminants per 1,000 days present (p=0.001), while recovery rate was maintained for Gramnegative organisms at 2.72 per 1,000 days
present compared to 3.41 (p=0.060), with no appreciable change in hospital-wide all-cause mortality (Figure 1). Overall, BCx stewardship efforts at the authors’ institution during a global BCx bottle shortage resulted in a 56% reduction in total BCxs drawn while retaining comparable positivity rates and all-cause mortality, including in the acute cancer care center for septic patients. Additionally, skin flora contaminant recovery was reduced by 49% while isolation of important pathogens like Gram-negatives was unchanged.

This study highlights that diagnostic stewardship efforts in a comprehensive cancer center can reduce low-yield blood culturing without compromising patient care and safety.2
References
1. US Food and Drug Administration (FDA). Disruptions in availability of BD BACTEC blood culture media bottles – letter to health care providers. 2022. Available at: https://web.archive.org/ web/20230311013824/https://www.fda.gov/medicaldevices/letters-health-care-providers/disruptionsavailability-bd-bactec-blood-culture-media-bottlesletter-health-care-providers. Last accessed: 3 November 2025.
2. Borjan J et al. Blood culture stewardship efforts at a comprehensive cancer center reduced isolation of skin flora contaminants without compromising patient care. Poster P-810. IDWeek, October 19-22, 2025.
Authors: *Kristen Kelly,1 Declan Quinn,1 Traci Leong,2 Jennifer Burns,1 Angelica Moran,3 Sophie Ginsberg,1 Rachel Baccile,4 Lilly Cheng-Immergluck1
1. Department of Pediatrics, University of Chicago Medicine, Illinois, USA
2. Emory University, Atlanta, Georgia, USA
3. Department of Pathology, University of Chicago Medicine, Illinois, USA
4. Center for Health and The Social Sciences, University of Chicago Medicine, Illinois, USA
*Correspondence to kristen.kelly2@uchicagomedicine.org
Disclosure: The authors have declared no conflicts of interest.
Keywords: Mycoplasma pneumoniae (MP), pneumonia, respiratory illness.
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:4749. https://doi.org/10.33590/microbiolinfectdisam/ ZUHW4840
Mycoplasma pneumoniae (MP) causes approximately two million infections in the USA,1 with increasing rates reported globally in the past few years.2-4 Rates in children have increased from 1.0 to 7.2% among those aged 2–4 years, and from 3.6 to 7.4% in those aged 5–17 years.1 In Illinois, USA, MP rates are about 5.1%, which is higher than rates of respiratory syncytial virus, influenza, or COVID-19.3 While MP usually presents as 'walking pneumonia' in schoolaged children, there have been increases in extrapulmonary manifestations. The authors propose a severity scoring system for MP based off of the Westley Croup Score (WCS) and Phoenix Sepsis Criteria (PSC).5 Both have assessments of neurologic status. The WCS assesses degrees of respiratory distress, while the PSC assesses vitals and state of inflammation.
To examine temporal trends in pediatric MP positivity rates, the authors aggregated counts, the total number of pediatric respiratory panel tests (PCR) ordered by year, and the count of positive MP results. Rates of MP positives per 1,000 tests were modeled using Poisson regression with the log of the number of respiratory panel tests included as an offset to adjust for variation in testing volume. Similar analyses describing time trends in MP hospitalization were not possible due to the infrequency of this event.
To identify factors associated with risk of being hospitalized for MP, the authors identified positive cases of MP in the electronic health record and conducted a chart review identifying demographics (sex, race, ethnicity, age), clinical findings (platelets, O2 requirements, respiratory distress defined as difficulty breathing, presence of retractions, tracheal tugging, head bobbing or grunting on physical exam, skin findings, and mental state), and year of infection. The authors used Chi-squared tests for categorical variables and t-tests for continuous variables. Factors significant on univariate analyses were included in multivariate analyses.
Statistical significance was defined as p<0.05. All analyses were conducted in R version 4.3.2.
The number of respiratory panels ordered, the number of MP cases, and the number of hospitalized MP cases all increased over the study period, though MP cases remained a small proportion of overall respiratory panels, peaking at 1.1% in 2024 (Table 1). At the start of the study period, the rate of positive
Table 1: Population characteristics pre- (2012–2019) and post- (2020–2024) COVID-19 pandemic.
Bolded p values indicate statistical significance (p<0.05).
BiPAP:
MP cases was approximately 1.3 per 1,000 respiratory panel tests. Over time, the rate increased by about 6.5% per year (incidence rate ratio: 1.065; 95% CI: 1.014–1.120), after adjusting for testing volume.
On univariate analysis, younger age, Black race, O2 requirements, respiratory support, altered mental state, and time-period (both pre- and post-COVID-19 pandemic and all other years versus 2024) were statistically significantly associated with greater odds of hospitalization. In multivariate analysis, Black race, respiratory distress, and time-period (both pre- and post-COVID-19 pandemic and all other years versus 2024) remained statistically significantly associated with greater odds of hospitalization.
Age, respiratory support, and altered mental status were all statistically significant predictors of MP severity. Younger, minority patients were more likely to be admitted.
2024 showed a statistically significant increase in both the number and severity of MP cases. More surveillance is needed in the USA to determine how prevalent and severe MP is in the pediatric population now and in the future.
References
1. Centers for Disease Control and Prevention (CDC). Mycoplasma pneumoniae infections have been increasing. 2024. Available at: https://www.cdc. gov/ncird/whats-new/mycoplasma-pneumoniaeinfections-have-been-increasing.html. Last accessed: 17 October 2025.
2. Bolluyt DC et al. Increased incidence of Mycoplasma pneumoniae infections and hospital admissions in the Netherlands, November to December 2023. Euro Surveill. 2024;29(4):2300724.
3. Urbieta AD et al. Mycoplasma pneumoniae at the rise not only in China: rapid increase of Mycoplasma pneumoniae cases also in Spain. Emerg Microbes Infect. 2024;13(1):2332680.
4. Yan C et al. Current status of Mycoplasma pneumoniae infection in China. World J Pediatr. 2024;20(1):1-4.
5. Kelly K et al. Development of a severity scoring system for Mycoplasma pneumoniae infection. Poster P-652. IDWeek, October 19-22, 2025.
Authors: *Sandhya Nagarakanti,1 Jamilah Shubeilat,1 Ali Abdulsahib,1 Natasha P. Dyal,1 Matt Bondi,2 Joseph Hentz,3 Erin Kaleta,4 Holenarasipur R. Vikram1
1. Division of Infectious Disease, Mayo Clinic Arizona, Phoenix, USA
2. Department of Internal Medicine, Mayo Clinic Arizona, Phoenix, USA
3. Department of Research and Biostatistics, Mayo Clinic Arizona, Phoenix, USA
4. Laboratory Medicine and Pathology, Mayo Clinic Arizona, Phoenix, USA
*Correspondence to nagarakanti.sandhya@mayo.edu
Disclosure: The authors have declared no conflicts of interest.
Keywords: Serology, strongyloides, treatment.
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:5051. https://doi.org/10.33590/microbiolinfectdisam/ JBBO6681
Strongyloides stercoralis is an intestinal parasitic nematode with a 2–7% prevalence in the USA. Immunosuppression can predispose to disseminated infection, concurrent bacterial sepsis, and significant morbidity and mortality. The authors sought to review positive Strongyloides serology (PSS) results across three academic medical centers.1,2
Electronic medical records of patients with PSS were reviewed between 2012–2023. Extracted data included demographics, country of origin, travel history, testing indication, clinical presentation, treatment regimen, and duration (Table 1). Patients were classified into chronic infection (PSS without additional clinical manifestations), hyperinfection syndrome (cutaneous, gastrointestinal tract, or lung involvement),
and disseminated infection (evidence of involvement of distant sites beyond hyperinfection, such as the brain, liver, kidneys, or bloodstream).
Overall PSS rate across the three sites was 5.5%. A total of 412 patients with PSS were identified, with a mean age of 56.7 years (SD±14.2), of whom 272 (66%) were male. The primary indication for testing was transplant evaluation in 230 (56%), eosinophilia workup in 84 (20%), suspicion of active infection in 74 (18%), travel history in 27 (7%), and pre-immunosuppression screening in 27 (7%). Four hundred and one (97.3%) were categorized as chronic infection, 10 (2%) as hyperinfection, and one (0.2%) as disseminated infection. Treatment was administered to 366 (89.3%) patients: 226 (61.7%) received two doses of ivermectin on consecutive days, 94 (25.7%) received a single dose, 26 (7.1%) received two doses repeated 2 weeks later, and 20 (5.5%) received alternative regimens. The patient with disseminated infection received 2 weeks of ivermectin followed by monthly dosing.
The majority of patients with PSS were asymptomatic, and treatment regimens were not uniform. Screening should be considered for patients currently receiving or initiating long-term immunosuppression that can potentiate Strongyloides dissemination. Developing a targeted testing algorithm based on epidemiologic factors, clinical suspicion, laboratory parameters, and the nature of immunosuppression can help identify at-risk patients who can receive pre-emptive therapy if infected.3,4
Table 1: Treatment regimens received.
MCA: Mayo Clinic Arizona; MCF: Mayo Clinic Florida; MCHS: Mayo Clinic Health System; MCR: Mayo Clinic Rochester.
References
1. Mayo Clinic Laboratories. Test definition: STRNG. 2025. Available at: https://www. mayocliniclabs.com/api/sitecore/TestCatalog/ DownloadTestCatalog?testId=63866. Last accessed: May 1 2025.
2. Nagarakanti S et al. Descriptive analysis of positive strongyloides serology across three academic medical centers. Poster P-1775. IDWeek, October 19–22, 2025.
3. Taheri B et al. Strongyloides stercoralis in the US military health system. Open Forum Infect Dis. 2023;10(3):ofad127.
4. Krolicki A, Nutman TB. Strongyloidiasis: a neglected tropical disease. Infect Dis Clin North Am. 2019;33(1):135-51.
Authors: Thomas Pustorino,1 Kelsey McManus,1 Nicholas Feola,1 *Abhay Dhand1
1. New York Medical College/Westchester Medical Center, Valhalla, USA *Correspondence to Abhay.dhand@wmchealth.org
Disclosure: Dhand has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Merck and Eurofins Viracor. The other authors have declared no conflicts of interest.
Keywords: Cardiotoxicity, cirrhosis, fluoroquinolone (FQ), personalized medicine, prolonged corrected QT (QTc), prophylaxis, risk analysis.
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:5254. https://doi.org/10.33590/microbiolinfectdisam/ ZZBF5679
Fluoroquinolone (FQ) antibiotics are currently the acceptable therapy for spontaneous bacterial peritonitis (SBP) prophylaxis in selected patients with cirrhosis.1 Based on additional comorbidities and fluid/electrolyte imbalance, these patients may have multiple other risk factors for prolonged corrected QT interval (QTc) beyond low-dose FQ therapy.2 Prolonged QTc can be associated with the risk of a life-threatening ventricular arrhythmia, torsades de pointes, which can lead to syncope, cardiac arrest, or sudden cardiac death.3-7
Consecutive adult patients with liver cirrhosis hospitalized from June 2022–June 2024 who were on FQ therapy for SBP prophylaxis for at least 72 hours during the inpatient
stay (regardless of outpatient therapy) with an available ECG were included in the study. Outcomes, measured retrospectively, included: the incidence of prolonged QTc (>450 ms), the number of cumulative risk factors associated with prolonged QTc in each patient while on FQ therapy, and any documented ventricular arrhythmia.
During the study period, 72 (36% female) patients with liver cirrhosis who met the study criteria were identified (Table 1). The majority of patients were on low dose ciprofloxacin (250 mg once a day). The average duration of FQ prophylactic therapy during hospitalization was 8.3 days (range: 3–30). The median age of patients was 53 years (interquartile range: 37–61). Among these 72 patients, concomitant risk factors for prolonged QTc were renal dysfunction (63%), hypokalemia (14%), hypomagnesemia (32%), azole therapy (2.8%), selective serotonin reuptake inhibitor therapy (9.7%), and diuretic therapy (26%). Cumulative risk factors (besides FQ therapy) for prolonged QTc were: one risk factor (22%), two risk factors(39%), three risk factors (28%), and ≥4 risk factors (11%). Prolonged QTc interval (>450 ms) was seen in 66% of the patients, with none having any evidence of ventricular arrhythmias.
Patients with liver cirrhosis are at risk of polypharmacy and organ dysfunction, resulting in multiple risk factors for prolonged QTc. While 66% of patients had evidence
Table 1: Incidence and cumulative risk factors for prolonged corrected QT interval during fluoroquinolone prophylaxis.
Patient characteristics, N (%) FQ
Mean duration of FQ prophylaxis
Concomitant medical conditions with risk of QTc prolongation, N (%)
Renal dysfunction (serum creatinine >1.5 mg/dL)
Hypokalemia (serum level <3 mEq/L)
Hypomagnesemia
Concomitant medications with risk of QTc prolongation, N (%)
days (range: 3–30)
(63%)
(14%)
Cumulative risk factors for prolonged QTc while on FQ prophylaxis, N (%)
1
(22) 2
3
Prolonged QTc present (>450 ms)
Evidence of ventricular arrhythmias (clinical or ECG)
FQ: fluoroquinolone; QTc: corrected QT interval.
(39)
(28)
(66)
of a prolonged QTc on routine ECG and had multiple other concomitant risk factors for prolonged QTc, none of the patients had evidence of any ventricular arrhythmias during their hospitalization. Prolonged QTc alone may not be considered an absolute contraindication for the use of FQs for SBP prophylaxis, when overall benefit is considered to be more than the risk. Active identification and treatment of potentially modifiable risk factors may help prevent any ventricular arrhythmias in patients with liver cirrhosis on FQ prophylaxis
References
1. Pustorino T et al. Incidence and cumulative risk factors for prolonged qtc interval in cirrhotic patients receiving fluoroquinolone prophylaxis. Poster P-863. IDWeek, October 19-22, 2025.
2. Moghnieh R et al. QTc prolongation during levofloxacin and triazole combination chemoprophylaxis: prevalence and predisposing
risk factors in a cohort of hematopoietic cell transplantation recipients. J Oncol Pharm Pract. 2022;29(3):534-42.
3. Choi EJ et al. Incidence and risk factors for QT prolongation associated with fluoroquinolones. J Korean Soc Health-Syst Pharm. 2023;40(2):195-204.
4. Berger FA et al. QTc prolongation during ciprofloxacin and fluconazole combination therapy: prevalence and associated risk factors. Br J Clin Pharmacol. 2018;84(2):369-78.
5. Lee W et al. Prolonged QT interval in cirrhosis: twisting time? Gut Liver. 2022;16(6):849-60.
6. Bernardi M et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with liver dysfunction and prognostic significance. Hepatology. 1998;27(1):28-34.
7. Bhardwaj A et al. QTc prolongation in patients of cirrhosis and its relation with disease severity. J Family Med Prim Care. 2020;9(6):3020-4.
In this interview, Barbara Trautner introduces the new Infectious Diseases Society of America (IDSA) complicated urinary tract infection (UTI) guideline presented at IDWeek 2025 by Trautner and colleagues from the IDSA complicated UTI Guideline Panel and the UTI WikiGuideline Panel. We learn how the updated uncomplicated/ complicated classifications and the four-step framework for empiric antibiotic selection can support more consistent, evidence-aligned decision-making in front-line care.
Featuring: Barbara Trautner


Barbara Trautner
Professor of Medicine, Co-Chief, Infectious Diseases Division, Washington University School of Medicine, St. Louis, Missouri, USA
I then volunteered to co-chair the IDSA UTI guidelines, and this experience has definitely been a career milestone for me
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:55-58. https://doi.org/10.33590/microbiolinfectdisam/QAJK1579
Q1What are the most practice-changing updates that were introduced in the session, “What’s New in Urinary Tract Infection (IDSA cUTI Guideline Panel and the UTI WikiGuideline Panel)”? Are there any that frontline clinicians should be applying immediately?
I think that the most practice changing update was for us to simply agree with clinical practice for the classifications of urinary tract infection (UTI). People were already using, in their minds, the distinction between uncomplicated and complicated UTI (cUTI) based on whether the infection has gone beyond the bladder or not, since that changes which antibiotics you're going to use and often the location of treatment, inpatient versus outpatient. We didn't invent
this idea; we just aligned our definitions with how people were practicing medicine. I think people were excited that we included the possibility that men could have an uncomplicated UTI because we all know what a burden it is to try to keep people on antibiotics longer than necessary. It is also practice changing that we included men.
Q2 Which parts of the guidance do you think will most improve real-world decision-making?
I really hope that our four-step process of choosing an empiric antibiotic for cUTI will help frontline clinicians. I'm going to give a shout out to panelists, Nico CortésPenfield [University of Nebraska Medical Center, Omaha, USA] and Kalpana Gupta [Veterans
Administration Boston Healthcare System; Boston University Chobanian & Avedisian School of Medicine, Massachusetts, USA], and everyone on the panel for helping us develop this concept. If we were to recommend three best drugs, that would be outdated in just a few years, and that recommendation might not even apply in certain places where they have different resistance patterns.
It's a four-step process that you apply to individualize the empiric antibiotic choice to the patient in front of you. You apply the four steps and then you choose among the remaining drugs that are in the preferred category, rather than just starting by giving everyone this one drug.
It’s codifying what people were already doing and making decisionmaking a little more formal for them. The first step is severity of illness, sepsis versus no sepsis, shock versus no shock. That decision puts you on different paths to preferred antibiotic groups. The next step is to consider what risk factors the patient has for an organism resistant to the antibiotic that you're thinking about. The third step is what antibiotic can the patient take? That’s kind of the art of medicine. Can they take oral or IV? What drug interactions may impact your choice? And the fourth step, only with septic patients, is to use the antibiogram to prevent excess mortality related to not choosing a broad enough antibiotic.
Q3What guidance emerged to help clinicians avoid overtreatment without compromising outcomes when balancing antimicrobial stewardship with patient expectations for rapid relief?
I don't think we're thinking so much in terms of rapid relief for cUTI, we're thinking of preventing mortality. It's a balance between antibiotic stewardship and preventing mortality, particularly in the septic patients where the risk of mortality is higher. We're very proud of the modeling that we did for the impact of choosing the wrong drug initially versus the right drug initially. That's how we were able to set our modeling thresholds for using the antibiogram in sepsis with shock, to choose an antibiotic for which the suspected organism is 90% likely to be susceptible to. In sepsis without shock, you're looking for an antibiotic for which the suspected organism has a predicted 80% susceptibility. There’s your balance, if you apply the antibiogram in the strictest sense to every single patient.
When we initially included the antibiogram step 4 in all cases, in a draft version of the recommendations, every patient ended up on a carbapenem when we applied it to real world cases. So there had to be some kind of balance. That's how we modeled the antibiogram use as a specific way to prevent excess mortality.
In the cUTI guidelines, we focused on cUTI empiric treatment, length of treatment, and IV-to-oral switch
Q4 What parts of UTI guidance still need development or updating?
In the cUTI guidelines, we focused on cUTI empiric treatment, length of treatment, and IV-to-oral switch. We're missing some pieces of UTI guidance like updating the uncomplicated UTI guidelines and then we probably need diagnostic guidelines because we don't touch on how you diagnose UTI in these guidelines. The WikiGuideline group, which we shared the session with, used a different methodology and was surprised by the lack of evidence for many key clinical questions in UTI. This is not a simple infection that should be neglected, it’s something that merits much more study, and I hope we can expand and do more guideline updates.
Q5 These guidelines represent a huge collaborative effort. Is there anyone you would like to recognize for their contributions?
First, the panelists who represented emergency medicine, hospital medicine, pharmacists, obstetrics, gynecology, and primary care. We wanted a representative panel of the people who would need to apply the guidelines. I want to thank all the panelists for all their intelligent suggestions that led us to the guidelines that we have. I particularly want to thank my cochair, Valéry Lavergne [Vancouver General Hospital, Canada], who is our GRADE methodologist, and who has an IDSA appointment to support guidelines development. The second and third authors, Nico Cortés-Penfield and Kal Gupta, spent a lot of time looking
To better design the research studies that need to be done, I think we need to gain people's trust by listening to what they have to say
at different revisions of the documents. Other people who made these better were our reviewers. Because we had so many different reviewers, different societies doing reviews, and public comments, all of that helped contribute and make the guidelines more practical. And, to the patients that participated as volunteers in all aspects of the guideline development, I am appreciative of their work.
Q6 What did it mean for you personally to present these new guidelines?
I'd be glad to share that. When I was an ID fellow, I was obsessed with the wonderful IDSA catheterassociated UTI guidelines. So much that Mac Hooten [University of Miami, Florida, USA], who was the lead author, let me review a draft and be one of their external reviewers. I'm actually named in the acknowledgments and that meant so much to me, as I had pored over every word. A few years later, I was invited to join the
IDSA Asymptomatic Bacteriuria Guidelines panel as a co-author, and that was super exciting. That project actually carried me through a period when I was being treated for breast cancer, as I had something very positive to work on and contribute to and that was meaningful. I then volunteered to co-chair the IDSA UTI guidelines, and this experience has definitely been a career milestone for me. Seeing them finally out and hearing how people are putting them into practice and exploring they can best make those guidelines fit their specific practice has been an incredibly meaningful experience.
Q7 Your long-standing research has focused on preventing recurrent catheterassociated urinary tract infections and reducing antimicrobial overuse. What strategies do you see as the most promising for reducing recurrence in high-risk populations?
I'm going to sound a little solemn here because I think we are
failing our patients in what we currently have available to prevent recurrence, because antibiotics are not preventing recurrence. A lot of the things sold on the Internet are not preventing recurrence, and if cranberry helps, and there's some evidence that it does, but it's a minor help. I think that we need a lot of strategies for preventing recurrence. There’s a big difference between with patients a catheter and those without a catheter.
For the “without catheter” population, people are predisposed to recurrent UTI. It's either something about their immune systems, their bladder mucosa adhesins, the specific organisms they get, or other things, but they keep getting these UTIs again and again. It's going to be multifactorial: vaccines are probably going to be one approach, urinary antiseptics will have a role, and bacteriophage, the living predators on bacteria, may have a role too. I'm envisioning multifaceted strategies to try to help prevent recurrent UTI.
Q8
How can clinicians better integrate patient education and behavioral interventions to improve UTI outcomes?
Clinicians need to listen to the patients. We had patient representatives who experience extreme forms of UTI symptoms. Their distress is real and their lives are impaired. The fullness of their life is impaired by this non-stop bladder pain. I think people can dismiss that and say, "No, it's not something they need antibiotics for, they’re just confused." We need to come together and listen to people. Our job is to relieve the suffering and find out what the suffering is caused by. To better design the research studies that need to be done, I think we need to gain people's trust by listening to what they have to say.
Q9
What are the current biggest gaps in equitable UTI management, given presentation, diagnostic access, and antimicrobial resistance patterns can vary across populations?
In our current well-resourced healthcare setting, when a woman or man gets an acute symptomatic UTI, even in the best of circumstances, they have trouble accessing care in a timely fashion.
If it happens at night, how do they get in and provide a urine specimen for culture? You don’t know if you can be seen the next morning. If I have trouble getting a urine culture for my own family member in a timely manner, how will someone who can't get to healthcare, doesn't have a regular doctor, or doesn't have insurance, actually get tested? That probably drives emergence of more pyelonephritis from untreated UTIs. That’s in the United States. In other parts of the world, antibiotics are sold over the counter, because otherwise, how will the person ever get any treatment? Then you're creating resistant organisms over time through self-treatment. People are empowered, they can have antibiotics when they need, but we don't know what they're treating, and then they get resistant organisms.
Q10
Which emerging areas excite you most from a translation-to-bedside perspective in the expanding field of UTIs?
I'm trying to start a bacteriophage trial for chronic bladder colonization with E. coli in persons with neurogenic bladders from spinal cord injury. I'm very interested in phage as an adjunct to antibiotics to prevent recurrent UTI, with the idea that the phage would help root out persistent organisms in different parts of the genitourinary
tract. The trial is registered with ClinicalTrials.gov (NCT06559618). We enrolled one patient before I moved institutions, but we plan to restart it here. It's for people with neurogenic bladders from spinal cord injury or spinal cord related disorders. It's a Phase I trial, so I'm not trying to relieve symptoms of UTI, just trying to see if we can get rid of the E. coli colonizing people’s bladders. This trial is funded by the Craig H. Neilsen Foundation.
Congratulations on your leadership appointment at Washington University! What do you hope to achieve in this role?
So, I'm at Washington University in St. Louis and I'm Co-Chief of Infectious Diseases. This is a thriving division, and I hope to take really good care of my people, the division and infectious diseases practitioners in general. I was motivated to make the switch for several reasons, one of which is I felt that infectious diseases as a field hasn't quite recovered from the pandemic. We got overworked, we got sad, and now we're dealing with people who don't want vaccines and we're sad about that too. It's a time to stand up and support infectious diseases practitioners, and I thought one of the best ways I could do that was in a leadership role of a strong division at a major academic institution.
It's a time to stand up and support infectious diseases practitioners, and I thought one of the best ways I could do that was in a leadership role of a strong division at a major academic institution
In this interview with Tina Tan, President of the Infectious Diseases Society of America (IDSA), we explore her vision for strengthening the infectious disease workforce, expanding vaccine access, and advancing paediatric infectious disease care. Tan outlines how IDSA aims to lead on evidence-based advocacy, multidisciplinary collaboration, and system-level impact in the years ahead.
Featuring: Tina Tan

Tina Tan
Pediatric Infectious Diseases
Attending, Ann & Robert H. Lurie Children’s Hospital of Chicago; President, Infectious Diseases Society of America (IDSA); Professor of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:59-60. https://doi.org/10.33590/microbiolinfectdisam/CCRG6511
Q1
What is your mission and vision for the Infectious Diseases Society of America (IDSA) during your tenure as President, and how do you see the organization advancing the field of infectious diseases in the coming years?
play in patient care, as well as helping healthcare colleagues recognize and understand the important role of pediatric infectious disease physicians.
There has also been a significant improvement in the care of infectious diseases for infants and children who are immunocompromised
My mission and vision for IDSA during my tenure as President have been to continue advocating strongly for the society's strategic priorities. These include building and sustaining a broad, diverse, valued infectious disease workforce; promoting the IDSA as the leader and trusted source for timely, evidence-based infectious disease information and expertise; and expanding infectious disease essays, leadership, and efforts to prevent, prepare for, and respond to infectious disease threats to protect our community. Also, I want to get more information about healthcare systems and the critically important role that infectious disease practitioners
Q2 You’ve spoken about the need for accurate and accessible vaccine information. What do you see as the biggest barriers to achieving equitable vaccine access?
The biggest barriers to achieving equitable vaccine access are numerous. They include limited healthcare services in different areas, geographic location and transportation issues, and the rapid spread of misinformation and disinformation about vaccines on social media. Medical mistrust and the cost of vaccines also play a great role.
Q3 How can the medical community better combat misconceptions surrounding vaccines in areas of lower trust in public health initiatives?
It's basically about listening to patients’ concerns about vaccines, addressing their questions empathetically, and providing them with scientifically sound information on the important benefits of vaccines. I then work with other individuals in the community whom the community members trust, such as religious leaders or other similar figures, to develop strong, scientifically sound messaging about the importance of vaccines and how they protect both individuals and their families.
Q4 What recent advancements or trends in pediatric infectious diseases are you currently most excited about?
So, the development of new vaccines and the optimization of antibiotic courses are necessary, because studies have shown that shorter courses are just as effective as some of the longer
courses still in use. There has also been a significant improvement in the care of infectious diseases for infants and children who are immunocompromised.
Lisa Akhtar, Assistant Professor, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA, does absolutely fantastic work on herpes simplex virus, which is really helpful because more individuals are now presenting with recurrent infections and require suppressive therapy.
Q5 With the rise of antibioticresistant infections, what strategies can pediatricians implement to balance effective treatment with responsible antibiotic use?
I think one of the major things is to work on making more accurate diagnoses of a patient's condition and not use antibiotics “just in case.” A lot of practitioners still do that. The key is to treat with the narrowest spectrum effective antibiotic and for the shortest duration recommended, so that antibiotics are not overused.
Q6 How can collaboration between pediatricians, infectious disease specialists, and public health officials be enhanced in a multidisciplinary team in addressing infectious disease outbreaks?
That's a really important question and one that is very relevant worldwide. We need to work together as a multidisciplinary team, using all healthcare providers' expertise and strengths to really address the different issues that arise during infectious disease outbreaks. I think what's really important is ensuring communication among all these individuals so that they can collaboratively develop a plan to basically help address all the major issues that arise with infectious disease outbreaks.
We need to work together as a multidisciplinary team, using all healthcare providers' expertise and strengths to really address the different issues that arise during infectious disease outbreaks
Author: *Karen C. Bloch1
1. Division of Infectious Diseases, Vanderbilt University Medical Center, Nashville, Tennessee, USA *Correspondence to karen.bloch@vumc.org
Disclosure: Bloch presented some of this information in Grand Rounds at NEOMeds April 2025 and received an honorarium for this talk; and is a Section Editor for ID portion, MKSAP, and the American College of Physicians (ACP).
Received: 10.12.25
Accepted: 11.11.25
Keywords: Anaplasmosis, babesiosis, ehrlichiosis, Ixodes scapularis, Lone Star tick, Lyme disease, Powassan virus, Rocky Mountain spotted fever, tickborne diseases United States, vector-borne infections.
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:61-69. https://doi.org/10.33590/microbiolinfectdisam/XZGH4742
The burden of tickborne diseases in the US more than doubled between 2004–2016, with >491,000 cases reported during this period.1 The reasons for the rising incidence of tickborne infections are complex and likely involve a combination of increased recognition and testing by clinicians, improved public health surveillance and reporting, expanding range of ticks due to evolving land use and climate change, and the recognition of new pathogens.2 Early recognition and initiation of directed antimicrobial treatment can be lifesaving.3-5 Thus, it is critical that clinicians working in primary care or urgent care settings consider a diagnosis of tickborne diseases in any patient with possible exposure and a compatible presentation. Knowledge of vector ticks is useful in determining the geographic distribution of diseases (Figure 1) and in considering the possibility of co-infection with a second tickborne agent. This article will focus on important updates in the diagnosis and management of patients in
the US with these increasingly common and potentially deadly infections.
Lyme disease, caused by infection with Borrelia burgdorferi sensu lato, or less commonly, B. mayonii, is the most common tick-borne infection in the US, with almost 90,000 cases reported to the Centers for Disease Control and Prevention (CDC) in 2023.7 Passive case reporting grossly underestimates the actual burden of disease. A study using commercial insurance claims estimated that the incidence of Lyme disease is 476,000 cases per year, or more than four-times the number reported to the CDC.8 More than 95% of all cases of Lyme disease occur in the Northeast, Mid-Atlantic, and Upper Midwest regions (Figure 1), areas where the blacklegged deer tick (Ixodes
1: Geographic distribution of selected tickborne diseases reported to the Centers for Disease control and Prevention (CDC), 2018.6
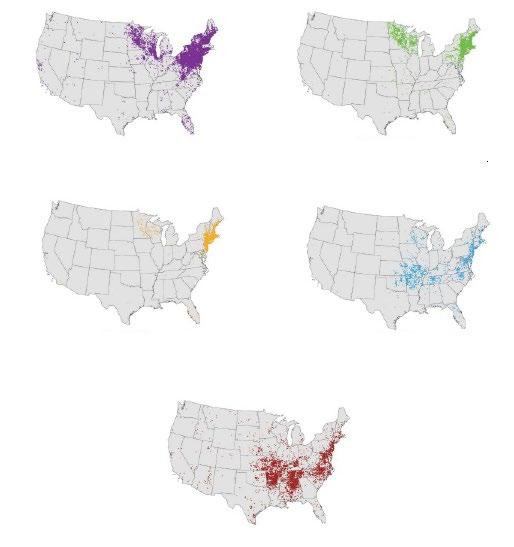
scapularis) is most abundant.2 The vector for Lyme disease on the West Coast is Ixodes pacificus or the western blacklegged tick. Notably, the number of counties with a high incidence of Lyme disease has increased by 300% since the 1990s.9
Transmission of B. burgdorferi to human hosts typically requires tick attachment for >24 hours.10 Symptom onset typically occurs 1–2 weeks after infection. Early localized disease manifests as erythema migrans (Figure 2), a target shaped or annular skin eruption at the site of the tick bite that is present in 60–80% of infections. Atypical presentations such as confluent erythema, sometimes with central ulceration, vesicles, or crusting are common,12,13 and a diagnosis of Lyme disease should be considered in any patient with potential exposure to Ixodes ticks presenting with a cutaneous lesion.
When erythema migrans is not present or not recognized, B. burgdorferi can spread, with disseminated disease occurring 2 weeks–6 months after the initial infection.14 This can present as fevers, multiple erythema migrans lesions at areas distinct from the initial inoculation (Figure 2), carditis (most commonly heart block), or neurologic disease, including cranial neuropathy, polyradiculopathy, or meningoencephalitis.15 Late disseminated disease usually occurs 6 months or later after infection and is characterized by oligoarticular migratory inflammatory arthritis.
The diagnosis and treatment of Lyme disease vary based on the stage of infection.15 Early infection may predate the development of antibodies, and therefore, for patients in Lyme endemic areas presenting with erythema migrans, a clinical diagnosis of Lyme disease can be made without laboratory confirmation. Treatment options for patients with erythema migrans and no visceral involvement include oral doxycycline for 10 days, or either amoxicillin or cefuroxime axetil for 14 days.
In the absence of typical erythema migrans, laboratory confirmation is recommended. Clinical Practice Guidelines developed jointly by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR) recommend using a two-tier serologic testing strategy.15 This involves an enzyme immunoassay (EIA) as the first step, and when this is equivocal or positive, a second test is performed for confirmation. With standard two-tier testing, the confirmatory test is a western blot. In 2019, the FDA approved the use of a second sequential EIA, the so-called modified two-tier testing strategy.16 Parenteral treatment with ceftriaxone or penicillin G is recommended for high-grade heart block or parenchymal neurologic disease; cranial neuropathy and meningitis respond to oral doxycycline therapy. Despite appropriate treatment, up to 20% of patients will develop post-treatment Lyme disease syndrome, characterized by fatigue, musculoskeletal pains, disordered sleep, and impaired cognition.17
Anaplasmosis is a bacterial infection caused by Anaplasma phagocytophilum that is also transmitted by the Ixodes tick. Coinfection with Lyme disease has been reported in >6% of anaplasmosis cases.18 Reported cases of anaplasmosis in the US have increased >16 fold between 2000–2019.19
Infection may be asymptomatic or associated with nonspecific symptoms such as fever (90%), headache (37%), and myalgias (42%).18 Symptom onset typically occurs between 1–2 weeks following the tick bite. Laboratory abnormalities, including leukopenia, thrombocytopenia, and elevated liver enzymes, support the diagnosis.
Morulae, basophilic inclusion bodies comprised of bacterial clusters, can be identified in the cytoplasm of neutrophils in >20% of cases,20 although the sensitivity of microscopy is highly dependent on the skill and persistence of the microscopist.21
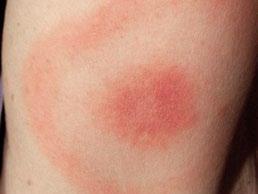
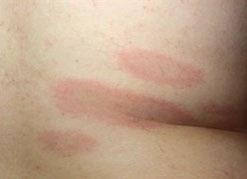
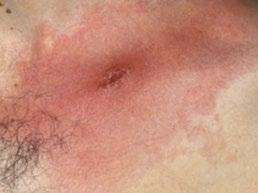
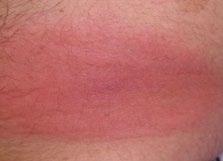
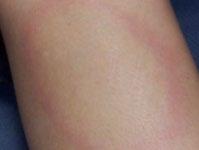
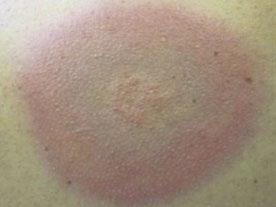

Definitive diagnosis of anaplasmosis is through either whole blood PCR or through serology, with seroconversion or a four-fold rise in titer between acute and convalescent serum diagnostic. Serologic testing is often falsely negative at presentation, as antibodies are only present in a minority of cases early in the course of infection. For this reason, treatment should not be delayed based on a negative acute antibody test.
Doxycycline is first-line therapy for anaplasmosis, with a duration of 10–14 days recommended to treat the possibility of incubating Lyme disease.22 Unlike older tetracyclines, doxycycline is not associated with staining of adult teeth and is indicated for all age groups.23,24 There is limited data to guide therapy for patients who have an absolute contraindication to doxycycline, but case reports have described cure with rifampin.25,26
Babesiosis is caused by intraerythrocytic protozoan parasites, most commonly Babesia microti, transmitted by the Ixodes tick. Coinfection with both Lyme disease and anaplasmosis has been reported.27 Babesiosis is most prevalent in coastal New England (Figure 1), but can occur throughout the range of the Ixodes tick. Infection can also be transmitted through blood transfusion, solid organ transplantation, or transplacental spread.28 In the former two settings, infection can occur in regions far outside the geographic range of the Ixodes tick.
Approximately 50% of B. microti infections are asymptomatic.29,30 Babesiosis most commonly presents as a nonspecific febrile illness, associated with headache and malaise. Mild hemolysis is the hallmark of infection, and findings of jaundice, scleral icterus, bilirubinuria, or anemia may support the diagnosis. Severe disease is predominantly seen in elderly or immunocompromised patients, including those with asplenia or HIV infection.31 Complications such as acute respiratory distress syndrome, disseminated intravascular coagulopathy, acute renal failure, congestive heart failure, splenic rupture, and sepsis may occur.28 Laboratory diagnosis of babesiosis is by visualization of intra-erythrocytic parasites on peripheral blood smear or through PCR testing.28 Serologic testing is confounded by the frequency of asymptomatic infection, and seropositivity may therefore simply denote prior infection. The preferred therapy for babesiosis is atovaquone plus azithromycin.32 Ancillary therapy with exchange transfusion is indicated for patients with parasitemia of >10% on blood smear or with severe hemolytic anemia.33
Powassan virus is an RNA flavivirus that is also spread by the Ixodes tick. The incidence of Powassan virus in the US is increasing, with most cases reported in the Great Lakes and Northeast regions.34-36 In contrast to Lyme disease, transmission of the Powassan virus can occur in as little as 15 minutes following tick attachment.37 Transmission by blood transfusion has also been reported.38
Symptomatic infection typically occurs within 5 weeks following a tick bite. Most infections present as a nonspecific febrile illness and remain undiagnosed. However, meningoencephalitis occurs in up to a quarter of infections, with a case fatality rate of 10%.39-41 Mortality may be higher in immunosuppressed patients.42,43
Powassan virus infection may be diagnosed serologically or by PCR. Diagnostic tests are not widely available commercially, but may be obtained through consultation with state/ territorial health departments when there is a clinical suspicion for infection. There is no effective antiviral therapy, and treatment is supportive care.34
Ehrlichiosis refers to human infection caused by Ehrlichia chaffeensis, E. ewingii, or E. muris eauclairensis. E. muris eauclairensis is an uncommon pathogen and, in contrast to the other causes of ehrlichiosis, is spread by the blacklegged deer tick E. chaffeensis, the most common cause of ehrlichiosis, is widely distributed in the Southeast and along the Eastern seaboard (Figure 1).
E. ewingii, also transmitted by the lone star tick, has a propensity to cause disease in immunocompromised hosts.19,44 The range of the vector tick is expanding, leading to an increased incidence of ehrlichiosis.45 Between 2019–2023, almost half of all cases reported in the US occurred in Arkansas, Missouri, New York, New Jersey, and North Carolina.46
Infection with Ehrlichia spp. commonly causes nonspecific symptoms, including fever, headache, and myalgias.47 A nonspecific macular or maculopapular rash is reported in up to 60% of cases, but primarily occurs in children.48 Laboratory abnormalities are important clues to the diagnosis, with leukopenia, thrombocytopenia, and elevated liver enzymes frequently present. Meningoencephalitis occurs in 5–10% of patients.47,49 Hemophagocytic lymphohistiocytosis may be a life-threatening complication of infection.50
The diagnosis of ehrlichiosis is suggested by visualization of morulae in white blood cell cytoplasm, although microscopy is extremely insensitive.47 The most sensitive diagnostic test for acute infection is whole blood PCR. Serology is useful for retrospective diagnosis when either seroconversion or a four-fold increase in titers is identified. Because only approximately 30% of cases have detectable antibodies at the time of presentation, empiric treatment with doxycycline should not be delayed pending serologic confirmation or stopped based on a negative acute titer. Increased mortality of ehrlichiosis in children45 has been attributed to provider hesitancy to prescribe doxycycline based on prior concerns that tetracyclines cause staining of adult teeth. This has now been disproven,23 and doxycycline is the preferred treatment for all age groups.
Heartland virus (HRTV), which was first identified in northwestern Missouri in 2009,51,52 is endemic to the Midwest and Eastern regions of the US, areas where the lone star tick is prevalent.
Asymptomatic or mild infection is believed to be common.53 Symptoms are identical to those of ehrlichiosis with fever, headache, and myalgias/arthralgias predominating. Similarly, leukopenia, thrombocytopenia, and elevated liver enzymes mimic ehrlichiosis. The distinguishing feature is that HRTV infection is not responsive to doxycycline.51 Fatal cases have been reported in immunocompromised patients or those with multiple comorbidities.54-57
Bourbon virus (BRBV) was first identified in an eastern Kansas farmer who died in 2014.58 Human infections with BRBV have been reported in the Midwest, although it is likely widespread throughout regions where the lone star tick is endemic.59-61
Symptoms of BRBV infection are nonspecific, including fever, fatigue, nausea/vomiting, and maculopapular rash. Laboratory abnormalities include leukopenia and thrombocytopenia. Although the clinical presentation mimics that of ehrlichiosis, similar to HRTV infection, doxycycline therapy is ineffective, and treatment is supportive care.
Both HRTV and BRBV can be diagnosed through serology or by RT-PCR. Testing can be performed through consultation with state or territorial health departments.
Spotted fever rickettsioses are a group of closely related tick-borne diseases that include Rocky Mountain spotted fever (RMSF), Rickettsia parkeri rickettsiosis, Pacific Coast tick fever, and rickettsialpox. These bacteria cross-react serologically and therefore are not easily differentiated by antibody testing. R. rickettsii, the bacteria causing RMSF, is the most common and virulent of the SFR.62
Historically, the highest incidence of RMSF has been in the Southeast and Southcentral US.63 Since 2003, the incidence of RMSF in the Southwestern US and Northern Mexico has increased significantly, leading to the recognition of the brown dog tick (Rhipicephalus sanguineus) as the vector in this region.64 Given the warmer climate in the Southwest, year-round transmission may occur in this region. A history of tick exposure is present in <60% of RMSF cases.65-67
RMSF is characterized by fever, headache, and rash. Early in the course of infection, this triad is present in a minority of cases.68 Specifically, the rash in RMSF, while ultimately present in >80% of patients, appears on average 2–5 days after the onset of fever.65,69 Rash may be absent or unrecognized in elderly or darkly pigmented individuals,70 which may lead to treatment delay and adverse outcome.69
Classically, the rash of RMSF initially presents with macular lesions around the wrists and ankles, followed by the development of diffuse petechiae that spread centrally but spares the face.63,71 Involvement of the palms and soles is noted in 58% of cases.66 Atypical cutaneous findings include pruritus, vesicular lesions, and urticaria.67 Laboratory abnormalities such as thrombocytopenia, elevated hepatic transaminases, and hyponatremia are commonly present.
Neurologic manifestations, including meningoencephalitis, are reported in as many as 40% of cases.62 CSF pleocytosis is often present with a neutrophilic predominance found in one-third of patients.62
References
1. Rosenberg R et al. Vital signs: trends in reported vectorborne disease cases—United States and territories, 2004–2016. MMWR Morb Mortal Wkly Rep. 2018;67(17):496-501.
2. Eisen RJ et al. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58(3):319-35.
Neuroimaging may provide an important clue to the diagnosis. The so-called “starry sky” pattern on MRI, characterized by multifocal diffusion restriction or T2 hyperintense lesions scattered throughout the subcortical and deep white matter, has been reported in both pediatric62,72,73 and adult patients.74
Confirmation of acute RMSF infection is primarily through serologic testing. Interpretation of a single elevated IgG antibody is problematic as this may reflect prior subclinical infection or infection with a cross-reactive pathogen.63,75 IgM antibodies are both insensitive and nonspecific.76 As with other tickborne infections, the initial antibody test is often negative, and therefore, treatment should not be delayed pending serologic confirmation of infection, or stopped based on a negative antibody result. Although not widely available, culture, immunohistochemical staining of skin lesions, or PCR may also be diagnostic.77
Treatment for RMSF in both children and adults is doxycycline given for ≥3 days after defervescence and for a minimum of 5–7 days.63 Longer courses may be indicated for CNS disease. A hallmark of RMSF is that patients typically defervesce within 24–48 hours of starting doxycycline.63,69 Delayed administration of doxycycline is a recognized risk factor for adverse outcomes.4,78,79 There are limited data regarding alternative treatments for patients with severe allergy to tetracyclines, and desensitization should be considered in this setting. Mortality of RMSF in the Southwest is 7–10%,63,69 significantly higher than in other endemic areas.
3. Mosites E et al. Knowledge, attitudes, and practices regarding Rocky Mountain spotted fever among healthcare providers, Tennessee, 2009. Am J Trop Med Hyg. 2013;88(1):162-6.
4. Zientek J et al. Self-reported treatment practices by healthcare providers could lead to death from Rocky Mountain spotted fever. J Pediatr. 2014;164(2):416-8.
5. Kuriakose K et al. Assessment of risk factors and outcomes of severe ehrlichiosis infection. JAMA Netw Open. 2020;3(11):e2025577.
6. Centers for Disease Control and Prevention (CDC). Tickborne diseases of the United States: a reference manual for healthcare providers. 2022. Available at: https://www.cdc.gov/ ticks/media/pdfs/2025/03/tickborne-
diseases-manual-508.pdf. Last accessed: November 13, 2025.
7. Centers for Disease Control and Prevention (CDC). Lyme disease surveillance and data. Available at: https://www.cdc.gov/lyme/dataresearch/facts-stats/surveillance-data-1. html. Last accessed: 1 October 2025.
8. Kugeler KJ et al. Estimating the frequency of Lyme disease diagnoses, United States, 2010–2018. Emerg Infect Dis. 2021;27(2):616-9.
9. Eisen L. Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick Borne Dis. 2018;9(3):535-42.
10. Kugeler KJ et al. Geographic distribution and expansion of human Lyme disease, United States. Emerg Infect Dis. 2015;21(8):1455-7.
11. Centres for Disease Control and Prevention (CDC). Lyme disease rashes. Available at: https://www.cdc.gov/lyme/ signs-symptoms/lyme-disease-rashes. html. Last accessed: 11 October 2025.
12. Smith RP et al. Clinical characteristics and treatment outcomes of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med. 2002;136(6):421-28.
13. Schotthoefer AM et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14(10):e30673.
14. Smith RP. Lyme disease. Ann Intern Med. 2025;178(5):ITC65-80.
15. Lantos PM et al. Clinical practice guidelines by the Infectious Diseases Society of America, American Academy of Neurology, and American College of Rheumatology: 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Neurology. 2021;96(6):262-73.
16. Centers for Disease Control and Prevention (CDC). Clinical testing and diagnosis for Lyme disease. Available at: https://www.cdc.gov/lyme/hcp/ diagnosis-testing/index.html. Last accessed: 1 October 2025.
17. Melia MT, Auwaerter PG. Time for a different approach to Lyme disease and long-term symptoms. N Engl J Med. 2016;374(13):1277-8.
18. Dumic I et al. Human granulocytic anaplasmosis—a systematic review of published cases. Microorganisms. 2022;10(7):1433.
19. Mowla SJ et al. Ehrlichiosis and anaplasmosis among transfusion and transplant recipients in the
United States. Emerg Infect Dis. 2021;27(11):2768-75.
20. Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2015;29(2):341-55.
21. Rand JV et al. Intracytoplasmic granulocytic morulae counts on confirmed cases of ehrlichiosis/ anaplasmosis in the Northeast. Am J Clin Pathol. 2014;141(5):683-6.
22. Centers for Disease Control and Prevention (CDC). Clinical care of anaplasmosis. Available at: https://www.cdc.gov/ anaplasmosis/hcp/clinical-care/index.html. Last accessed: 11 October 2025.
23. Todd SR et al. No visible dental staining in children treated with doxycycline for suspected Rocky Mountain spotted fever. J Pediatr. 2015;166(5):1246-51.
24. Ravindra D et al. Antibiotic exposure and dental health: a systematic review. Pediatrics. 2023;152(1):e2023061350.
25. MacQueen D, Centellas F. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2022;36(3):639-54.
26. Dhand A et al. Human granulocytic anaplasmosis during pregnancy: case series and literature review. Clin Infect Dis. 2007;45(5):589-93.
27. Caulfield AJ, Pritt BS. Lyme disease coinfections in the United States. Clin Lab Med. 2015;35(4):827-46.
28. Waked R, Krause PJ. Human babesiosis. Infect Dis Clin North Am. 2022;36(3):655-70.
29. Krause PJ. Babesiosis diagnosis and treatment. Vector Borne Zoonotic Dis. 2003;3(1):45-51.
30. Bloch EM et al. Persistence of Babesia microti infection in humans. Pathogens. 2019;8(3):102.
31. Heller HM. Babesiosis in immunosuppressed hosts: pathogenesis, diagnosis and management. Curr Opin Infect Dis. 2024;37(5):327-32.
32. Krause PJ et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA): 2020 guideline on diagnosis and management of babesiosis. Clin Infect Dis. 2021;72(2):185-9.
33. O’Bryan J et al. Parasite burden and red blood cell exchange transfusion for babesiosis. J Clin Apher. 2021;36(1):127-34.
34. Khan M et al. An overview of Powassan virus disease. Neurohospitalist. 2019;9(4):181-2.
35. Centers for Disease Control and Prevention (CDC). Powassan virus, historic data. Available at: https://www.cdc.gov/ powassan/data-maps/historic-data.html. Last accessed: 10 October 2025.
36. Krow-Lucal ER et al. Powassan virus disease in the United States, 2006–2016. Vector Borne Zoonotic Dis. 2018;18(6):286-90.
37. Ebel GD, Kramer LD. Short report: duration of tick attachment required for transmission of Powassan virus by deer ticks. Am J Trop Med Hyg. 2004;71(3):268-71.
38. Taylor L et al. Powassan virus infection likely acquired through blood transfusion presenting as encephalitis in a kidney transplant recipient. Clin Infect Dis. 2021;72(6):1051-4.
39. Vahey GM et al. Seroprevalence of Powassan virus infection in an area experiencing a cluster of disease cases: Sussex County, New Jersey, 2019. Open Forum Infect Dis. 2022;9(3):ofac023.
40. Piantadosi A et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis. 2016;62(6):707-13.
41. Picheca C et al. Polio-like manifestation of Powassan virus infection with anterior horn cell involvement, Canada. Emerg Infect Dis. 2019;25(8):1609-11.
42. Solomon IH et al. Fatal Powassan encephalitis (deer tick virus, lineage II) in a patient with fever and orchitis receiving rituximab. JAMA Neurol. 2018;75(6):746-50.
43. Kapadia RK et al. Severe arboviral neuroinvasive disease in patients on rituximab therapy: a review. Clin Infect Dis. 2023;76(6):1142-8.
44. Thomas LD et al. Human ehrlichiosis in transplant recipients. Am J Transplant. 2007;7(6):1641-7.
45. Nichols Heitman K et al. Increasing incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med Hyg. 2016;94(1):52-60.
46. Centers for Disease Control and Prevention (CDC). Ehrlichiosis epidemiology and statistics. Available at: https://www.cdc.gov/ehrlichiosis/ data-research/facts-stats/index.html. Last accessed: 10 October 2025.
47. Gygax L et al. Human monocytotropic ehrlichiosis—a systematic review and analysis of the literature. PLoS Negl Trop Dis. 2024;18(8):e0012377.
48. Schutze GE et al.; Tick-borne Infections in Children Study (TICS) Group. Human monocytic ehrlichiosis in children. Pediatr Infect Dis J. 2007;26(6):475-9.
49. Iyamu O et al. Neurological manifestations of ehrlichiosis among a cohort of patients: prevalence and clinical symptoms. BMC Infect Dis. 2024;24(1):701.
50. Hammoud K et al. Ehrlichiosisassociated hemophagocytic lymphohistiocytosis: a case series and review of the literature. Case Rep Hematol. 2023;2023:5521274.
51. Pastula DM et al. Notes from the field: Heartland virus disease—United States, 2012–2013. MMWR Morb Mortal Wkly Rep. 2014;63(12):270-1.
52. Staples JE et al. Investigation of Heartland virus disease throughout the United States, 2013–2017. Open Forum Infect Dis. 2020;7(5):ofaa125.
53. Lindsey NP et al. Seroprevalence of Heartland virus antibodies in blood donors, northwestern Missouri, USA. Emerg Infect Dis. 2019;25(2):358-60.
54. Liu S et al. Fatal case of Heartland virus disease acquired in the Mid-Atlantic region, United States. Emerg Infect Dis. 2023;29(5):992-6.
55. Hevey MA et al. Heartland virus infection in a heart transplant recipient from the Heartland. Transpl Infect Dis. 2019;21(4):e13098.
56. Carlson AL et al. Heartland virus and hemophagocytic lymphohistiocytosis in immunocompromised patient, Missouri, USA. Emerg Infect Dis. 2018;24(5):893-7.
57. Fill MA et al. Novel clinical and pathologic findings in a Heartland virus-associated death. Clin Infect Dis. 2017;64(4):510-2.
58. Kosoy OI et al. Novel thogotovirus associated with febrile illness and death, United States, 2014. Emerg Infect Dis. 2015;21(5):760-4.
59. Dupuis AP 2nd et al. Bourbon virus transmission, New York, USA. Emerg Infect Dis. 2023;29(1):145-8.
60. Egizi A et al. Lone star ticks (Acari: Ixodidae) infected with Bourbon virus in New Jersey, USA. J Med Entomol. 2023;60(4):842-6.
61. Zychowski DL et al. Evidence of human Bourbon virus infections, North Carolina, USA. Emerg Infect Dis. 2024;30(11):2396-9.
62. Bradshaw MJ et al. Meningoencephalitis due to spotted fever rickettsioses, including Rocky Mountain spotted fever. Clin Infect Dis. 2020;71(1):188-95.
63. Biggs HM et al. Diagnosis and management of tick-borne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65(2):1-44.
64. Demma LJ et al. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353(6):587-94.
65. Gottlieb M et al. The evaluation and management of Rocky Mountain spotted fever in the emergency department: a review of the literature. J Emerg Med. 2018;55(1):42-50.
66. Jay R et al. Clinical characteristics of Rocky Mountain spotted fever in the United States: a literature review. J Vector Borne Dis. 2020;57(2):114-20.
67. Traeger MS et al. Rocky Mountain spotted fever characterization and comparison to similar illnesses in a highly endemic area—Arizona, 2002–2011. Clin Infect Dis. 2015;60(11):1650-8.
68. Walker DH et al. Rickettsiosis subcommittee report to the tick-borne disease working group. Ticks Tick Borne Dis. 2022;13(1):101855.
69. Regan JJ et al. Risk factors for fatal outcome from Rocky Mountain spotted fever in a highly endemic area— Arizona, 2002–2011. Clin Infect Dis. 2015;60(11):1659-66.
70. Helmick CG et al. Rocky Mountain spotted fever: clinical, laboratory, and epidemiological features of 262 cases. J Infect Dis. 1984;150(4):480–8.
71. Sexton DJ et al. Rocky Mountain spotted fever. Med Clin North Am. 2002;86(2):351-60, vii–viii.
72. Rhodes SD et al. Brain death secondary to Rocky Mountain spotted fever encephalitis. Case Rep Crit Care. 2020;2020:5329420.
73. Crapp S et al. Rocky Mountain spotted fever: ‘starry sky’ appearance with diffusion-weighted imaging in a child. Pediatr Radiol. 2012;42(4):499-502.
74. Mikhaiel JP et al. Rocky Mountain spotted fever encephalitis and “starry sky” pattern on MRI: a case report. Neurologist. 2025;30(1):34-8.
75. Straily A et al. Antibody titers reactive with Rickettsia rickettsii in blood donors and implications for surveillance of spotted fever rickettsiosis in the United States. J Infect Dis. 2020;221(8):1371-8.
76. McQuiston JH et al. Inadequacy of IgM antibody tests for diagnosis of Rocky Mountain spotted fever. Am J Trop Med Hyg. 2014;91(4):767-70.
77. Drexler NA et al. National surveillance of spotted fever group rickettsioses in the United States, 2008–2012. Am J Trop Med Hyg. 2016;94(1):26-34.
78. Alvarez-Hernandez G et al. Clinical profile and predictors of fatal Rocky Mountain spotted fever in children from Sonora, Mexico. Pediatr Infect Dis J. 2015;34(2):125-30.
79. Drexler NA et al. Morbidity and functional outcomes following Rocky Mountain spotted fever hospitalization— Arizona, 2002–2017. Open Forum Infect Dis. 2022;9(10):ofac506.
Authors: *Galo Guillermo Farfán Cano,1-4 Ranjith Kumar Nanda Kumar,5 Soumiya Nadar,5 Anthony Sfeir,6 Tishya Mukherjee7
1. School of Medicine, University of Guayaquil, Ecuador
2. Society of Infectious Diseases of Guayas, Guayaquil, Ecuador
3. Intermediate Care Unit, IESS Los Ceibos Hospital, Guayaquil, Ecuador
4. King Juan Carlos University, Madrid, Spain
5. School of Medicine, Tbilisi State Medical University, Georgia
6. Lebanese University, Beirut, Lebanon
7. Nicolae Testemițanu State University of Medicine and Pharmacy, Chișinău, Moldova
*Correspondence to dr.galo.farfan.cano@gmail.com
Disclosure: The authors have declared no conflicts of interest.
Received: 02.19.25
Accepted: 09.25.25
Keywords: Bats, climate change, cutaneous histoplasmosis, Histoplasma capsulatum, histoplasmosis, HIV, immunocompromised, non-endemic regions.
Citation: Microbiol Infect Dis AMJ. 2025; 3[1]:70-75. https://doi.org/10.33590/microbiolinfectdisam/UJBT5845
Histoplasmosis is a fungal infection caused by Histoplasma capsulatum, 1-3 a dimorphic fungus1,3-6 belonging to the family Ajellomycetaceae, 7 also known as Darling’s disease.4 It most frequently affects people living with HIV (PLWH).2,3,6
New hyperendemic areas have emerged in the Po River basin in Italy and various parts of India, where the prevalence of HIV among patients with histoplasmosis ranges from 2–29%.8-10 Reports from South Asia, including Bangladesh, Nepal, Pakistan, and Sri Lanka, also highlight a growing disease burden owing to improved healthcare access and diagnostics.10-12 Although globally distributed, H. capsulatum is most prevalent in Africa and the Americas.3,6,13-15 In recent years,
cases have increasingly been reported in non-endemic regions, a trend attributed to enhanced diagnostic capabilities, increased physician awareness, climate change, and the global spread of HIV.8,16-18
Among PLWH in endemic areas, the annual incidence of progressive disseminated histoplasmosis is approximately 5%, with persistently high mortality despite widespread availability of antiretroviral therapy (ART).2,10,11 A USA-based retrospective study (2002–2017) revealed a 37% overall mortality among PLWH diagnosed with histoplasmosis,11,19 underscoring the critical need for timely recognition and intervention.
Cutaneous histoplasmosis, while infrequent, serves as a meaningful indicator of disseminated disease in patients who
are immunocompromised.6,9,20 The fungus thrives in soils contaminated with avian and chiropteran excreta,7,21-25 commonly found in old buildings, caves, and humid environments. Inhaled spores are phagocytosed by alveolar macrophages, initiating infection. While healthy individuals mount an adaptive immune response that limits disease progression, PLWH lack sufficient cellular immunity, allowing for fungal proliferation and hematogenous dissemination to various organs, including the skin.
This article provides an overview of cutaneous histoplasmosis in PLWH, highlighting its occurrence in non-endemic areas and the challenges in diagnosis.
H. capsulatum includes human-specific variants such as H. capsulatum var. capsulatum and H. capsulatum var. duboisii, as well as recently described species like H. suramericanum and H. mississippiensis This fungus thrives in environments such as soil contaminated with bat or bird feces, old buildings, and humid areas with high rainfall. The primary mode of entry into the host is inhalation of microconidia.1,2,7,11,22,23,25
Once inhaled, microconidia are phagocytosed by alveolar macrophages. Bats serve as primary reservoirs and dispersers of Histoplasma, although the fungus is also found in other wild and domestic animals.25-28
Climate change is expanding the geographic range of Histoplasma and its vectors, raising concerns about new endemic zones and evolving disease patterns.29-33
In immunocompetent individuals, infection is often asymptomatic or presents as selflimited pneumonia.34-38 Conversely, patients who are immunocompromised, especially those with HIV, are at higher risk for hematogenous dissemination with multi-organ involvement, including spleen, liver, bone marrow, central nervous system (CNS), and skin (Figure 1).2,3,6,8,9,15,18,19,20,35
Cutaneous histoplasmosis occurs in 10–25% of patients with AIDS with disseminated histoplasmosis.39-41 Lesions are categorized as primary or secondary. Primary cutaneous histoplasmosis is rare and results from direct inoculation of the fungus into the skin, often through trauma, leading to localized lesions such as nodules, ulcers, or verrucous plaques.6,20,36,37 Secondary cutaneous histoplasmosis occurs in up to 17% of patients with disseminated disease, particularly among individuals who are immunocompromised (e.g., those with HIV/ AIDS, organ transplant recipients, or those on immunosuppressive therapy).42-45
Manifestations include generalized skin lesions such as maculopapular eruptions, nodules, plaques, ulcers, or crusted papules. These lesions are often nonpruritic but may ulcerate or become painful in chronic cases. They typically affect the face, trunk, and extremities, but can appear anywhere. Mucosal involvement (e.g., oral or nasal ulcers) often indicates systemic dissemination.6,20,36-38,40,44-46
Diagnosis relies on histopathological examination, which typically reveals intracellular yeast forms within macrophages.6,33,39,40,45,47 Although direct microscopy or skin scraping can provide rapid preliminary information, over-reliance on these methods is discouraged due to their low specificity and potential for misidentification. Confirmatory testing using culture, histopathology, or antigen detection remains essential for accurate diagnosis. Cultures confirm the diagnosis, though results may take weeks.6,48-50 Molecular diagnostics and antigen detection, as claims, have improved sensitivity, particularly in endemic areas.50-52
Histoplasmin skin tests are available but lack specificity.15,53 Urine antigen detection is under study.54,55 Presence of cutaneous lesions

Bird and bat droppings

Histoplasma capsulatum
Exposure and inhalation of microconidia
Pulmonary containment in healthy host
HIV-induced immunosuppresion
Cutaneous and mucosal involvement
Mucosal lesions
Disseminated histoplasmosis in immunocompromised host
Histoplasma capsulatum spores from contaminated soil are inhaled and phagocytosed by alveolar macrophages. In immunocompetent hosts, infection remains localized, and in individuals infected by HIV, impaired cellular immunity allows hematogenous dissemination to multiple organs, including the skin and mucous membranes. Cutaneous lesions may present as papules, nodules, ulcers, or plaques, frequently indicating systemic disease.
in patients who are immunocompromised warrants a high index of suspicion for disseminated histoplasmosis, underscoring the importance of early diagnosis and treatment to reduce mortality.
In patients with HIV, management of cutaneous histoplasmosis aligns with that of disseminated histoplasmosis. Treatment is generally divided into induction and maintenance phases.
• Induction therapy: for severe or disseminated histoplasmosis, especially in patients who are immunocompromised, liposomal amphotericin B is the preferred agent, and is administered intravenously at 3 (AI)–5 (AIII) mg/kg/day for 2 weeks.3,6,16,49,56-58
• Maintenance therapy: this phase aims to rapidly reduce fungal burden. When amphotericin B is not tolerated, itraconazole is used as an alternative, although it is less effective in severe cases. Standard dosing consists of 200 mg every 8 hours for the first 3 days, followed by 200 mg every 12 hours for 12 weeks.59-61 If itraconazole is not tolerated, fluconazole or posaconazole may be considered, although these agents are less potent.3,6,57,59-62
For PLWH, initiation of ART is essential to restore immune function, enhancing antifungal treatment efficacy and preventing relapse. In cases of severe inflammatory response, corticosteroids may be used temporarily alongside antifungal therapy to manage symptoms such as edema and erythema. Surgical excision may be
considered for refractory or localized lesions as adjunctive therapy. Regular monitoring of fungal antigen levels (e.g., H. capsulatum polysaccharide antigen) is critical to assess treatment efficacy. Itraconazole has limited effectiveness in CNS involvement due to poor cerebrospinal fluid penetration. Suspected CNS histoplasmosis requires alternative therapeutic strategies.14,20,62
Cutaneous histoplasmosis is a frequent manifestation of disseminated disease in PLWH, signaling systemic involvement with high morbidity and mortality if untreated. Early diagnosis using histopathology, culture, and antigen detection is critical.
Treatment involves initial liposomal amphotericin B followed by prolonged itraconazole maintenance alongside timely antiretroviral therapy to restore immunity. Management of complications and monitoring of fungal antigen levels improve outcomes. With expanding geographic distribution, increased awareness and prompt intervention are essential to reduce mortality.
References
1. Araúz AB, Papineni P. Histoplasmosis. Infect Dis Clin North Am. 2021;35(2):471-91.
2. Farfán-Cano GG et al. Approach to the diagnosis of pulmonary opportunistic infections in adults with AIDS. InterAm J Med Health. 2021;DOI:10.31005/iajmh. v4i.169.
3. Farfán-Cano GG et al. Disseminated histoplasmosis in a patient with HIV infection. INSPILIP. 2018;DOI:10.31790/ inspilip.v2i2.99.
4. Mahajan M. Etymologia: histoplasma capsulatum. Emerg Infect Dis. 2021;27(3):969.
5. Kontogiannis D et al. Histoplasmosis in patients living with HIV in Europe: review of literature. Front Microbiol. 2024;15:1418530.
6. Farfán-Cano GG et al. Cutaneous histoplasmosis. A case report. Microbes Infect. Chemother. 2022;2:e1353.
• Cutaneous histoplasmosis occurs in 10–25% of patients with HIV with disseminated disease.
• Lesions include nodules, plaques, ulcers, and maculopapular eruptions, often indicating systemic spread.
• Diagnosis relies on histopathology, culture, molecular assays, and antigen detection.
• Liposomal amphotericin B is the preferred induction therapy. Itraconazole is used for maintenance.
• ART initiation is crucial for immune recovery and treatment success.
• Itraconazole has limited efficacy in CNS involvement. Alternative treatments are necessary.
• Climate change and HIV spread are expanding histoplasmosis to new geographic areas, requiring heightened clinical awareness.
7. de Perio MA et al. Occupational histoplasmosis: epidemiology and prevention measures. J Fungi. 2021;7(7):510.
8. Ravindra A et al. 835. Histoplasmosis in Western India: shedding light on a potentially underdiagnosed disease – a prospective study from a tertiary care centre. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.880.
9. Arora S et al. Disseminated histoplasmosis in HIV patients- case series from a single tertiary care centre in India. APIK J Int Med. 2022;10(2):98102.
10. Ashraf N et al. Re-drawing the maps for endemic mycoses. Mycopathologia. 2020;185(5):843-65.
11. Efrim D et al., “Epidemiology of histoplasmosis,” Dantes E, Dumea E (eds.), Histoplasmosis - A Comprehensive Study of Epidemiology, Pathogenesis, Diagnosis, and Treatment (2023), IntechOpen.
12. Sigera LSM et al. P286 Histoplasmosis in Sri Lanka. Med Mycol. 2022;60(Suppl 1):myac072P286.
13. Amona FM et al. Histoplasmosis in the Republic of Congo dominated by African histoplasmosis, histoplasma capsulatum var. duboisii. PLoS Negl Trop Dis. 2021;15(5):e0009318.
14. Escalonilla P et al. Disseminated histoplasmosis with skin involvement in an HIV-infected patient. Actas Dermosifiliogr. 1999;90(11):591-4.
15. Cáceres DH et al. Tackling Histoplasmosis infection in people living with HIV from Latin America: from diagnostic strategy to public health solutions. J Fungi. 2023;9(5):558.
16. Mohseni Afshar Z et al. A comprehensive review on HIVassociated dermatologic manifestations: from epidemiology to clinical management. Int J Microbiol. 2023;2023:6203193.
17. Kontogiannis C et al. Electrical storm: current evidence, clinical implications, and future perspectives. Curr Cardiol Rep. 2019;21(9):96.
18. Lopez A et al. Successful use of multidisciplinary palliative care in the outpatient treatment of disseminated histoplasmosis in an HIV positive child. Children (Basel). 2021;8(4):273.
19. Cherabie J et al. Long-term mortality after histoplasma infection in people with HIV. J Fungi (Basel). 2021;7(5):369.
20. Vasudevan B et al. Primary cutaneous histoplasmosis in a HIVpositive individual. J Glob Infect Dis. 2010;2(2):112.
21. Meredith TA, Ulrich JN, “Infectious Endophthalmitis,” Retina (2013) 5th edition, Philadelphia: Elsevier, pp.2019-39.
22. Diaz JH. Environmental and wildernessrelated risk factors for histoplasmosis: more than bats in caves. Wilderness Environ Med. 2018;29(4):531-40.
23. Sudnik P et al. Histoplasmosis associated with bat guano exposure in cannabis growers: 2 cases. Open Forum Infect Dis. 2024;11(12):ofae711.
24. Gugnani HC, Denning DW. Infection of bats with Histoplasma species. Med Mycol. 2023;61(8):myad080.
25. Chatterjee SS et al., “Histoplasmosis,” Parija SC, Rudramurthy SM (eds.), Textbook of Fungal Zoonoses and Sapronoses (2024). Springer Nature, pp.251-68.
26. Ray SC, Rappleye CA. Flying under the radar: histoplasma capsulatum avoidance of innate immune recognition. Semin Cell Dev Biol. 2019;89:91-8.
27. Valdez AF et al. Pathogenicity & virulence of histoplasma capsulatum - a multifaceted organism adapted to intracellular environments. Virulence. 2022;13(1):1900-19.
28. Mittal J et al. Histoplasma capsulatum: mechanisms for pathogenesis. Curr Top Microbiol Immunol. 2019;422:157-91.
29. van Rhijn N, Bromley M. The consequences of our changing environment on life threatening and debilitating fungal diseases in humans. J Fungi (Basel). 2021;7(5):367.
30. Salazar-Hamm P, Torres-Cruz TJ. The impact of climate change on human fungal pathogen distribution and disease incidence. Curr Clin Microbiol Rep. 2024;11(3):140-52.
31. Seidel D et al. Impact of climate change and natural disasters on fungal infections. Lancet Microbe. 2024;5(6):e594-605.
32. Taylor ML et al. Considerations about the geographic distribution of histoplasma species. Appl Environ Microbiol. 2022;88(7):e02010-21.
33. CDC. Histoplasma in the environment: an overview. Histoplasmosis. 2024. Available at: https://www.cdc.gov/niosh/ histoplasmosis/about/environment.html. Last accessed: 22 December 2024.
34. Agarwal M et al. Progressive disseminated histoplasmosis in an immunocompetent host: a rare presentation of an uncommon disease. Acta Biomed. 2021;92(S1):e2021134.
35. Araújo MRB et al. Oral presentation of histoplasmosis in non-HIV immunocompromised patient after cardiac transplant: first Brazilian case report. Rev Soc Bras Med Trop. 2023;56:e0499-2022.
36. Avila RM et al. Case report: primary cutaneous histoplasmosis in an immunocompetent patient after cosmetic injection of platelet-rich plasma treated with trimethoprim-sulfamethoxazole. Am J Case Rep. 2024;25:e942660.
37. Batista JM et al. Primary cutaneous histoplasmosis difficult to treat in immunocompetent patient: case report and literature review. Einstein (Sao Paulo). 2021;19:eRC5488.
38. Gómez-Santana LV et al. Mucocutaneous manifestations of infection by histoplasma capsulatum in HIVnegative immunosuppressed patients. Actas Dermosifiliogr (Engl Ed). 2018;109(4):e27-32.
39. Chang P, Rodas C. Skin lesions in histoplasmosis. Clin Dermatol. 2012;30(6):592-8.
40. Koley S et al. Disseminated cutaneous histoplasmosis, an initial manifestation of HIV, diagnosed with fine needle aspiration cytology. Indian J Dermatol. 2014;59(2):182.
41. Oladele RO et al. Prevalence of histoplasmosis among persons with advanced HIV disease, Nigeria. Emerg Infect Dis. 2022;28(11):2269-77.
42. Linder KA, Kauffman CA. Histoplasmosis: epidemiology, diagnosis, and clinical manifestations. Curr Fungal Infect Rep. 2019;13(3):120-8.
43. Saullo JL, Miller RA. Updates on histoplasmosis in solid organ transplantation. Curr Fungal Infect Rep. 2022;16(4):165-78.
44. Romo Erazo JM et al. Primary cutaneous histoplasmosis in immunocompetent patients. Report of 2 cases. AVFT. 2019;38(2):19-21.
46. Cortez-Vila JA et al. Disseminated cutaneous histoplasmosis and its recurrence in an apparently immunocompetent patient. Cureus. 2024;16(5):e60433.
47. Panuganti S et al. A rare case of disseminated cutaneous histoplasmosis. Indian J Dermatol Venereol Leprol. 2022;88:533-6.
48. Buitrago MJ, Valero C, “Laboratory diagnosis of histoplasmosis: an update,” Bongomin F (ed.), Histoplasma and Histoplasmosis (2020), IntechOpen.
49. Azar MM et al. Current concepts in the epidemiology, diagnosis, and management of histoplasmosis syndromes. Semin Respir Crit Care Med. 2020;41(1):013-030.
50. Azar MM, Hage CA. Laboratory diagnostics for histoplasmosis. J Clin Microbiol. 2017;55(6):1612-20.
51. Martínez-Gamboa A et al. Diagnostic accuracy of antigen detection in urine and molecular assays testing in different clinical samples for the diagnosis of progressive disseminated histoplasmosis in patients living with HIV/AIDS: a prospective multicenter study in Mexico. PLoS Negl Trop Dis. 2021;15(3):e0009215.
52. Villareal K et al. The current and future states of diagnostic tests for histoplasmosis with a focus on people with HIV and disseminated histoplasmosis. J Fungi (Basel). 2023;9(8):793.
53. Bennett J. Concerning features of emerging fungal infections. Physician Assist Clin. 2023;8(3):433-52.
54. Kuate MPN et al. Screening for acute disseminated histoplasmosis in HIV disease using urinary antigen detection enzyme immunoassay: a pilot study in Cameroon. J Microbiol Methods. 2021;185:106226.
55. Kuate MPN et al. Diagnosing disseminated histoplasmosis in advanced HIV/AIDS disease in Cameroon using a point of care lateral flow assay. Ther Adv Infect Dis. 2022;9:20499361221132133.
56. Murray M, Hine P. Treating progressive disseminated histoplasmosis in people living with HIV. Cochrane Database Syst Rev. 2020;(4):CD013594.
45. Raina RK et al. Primary cutaneous histoplasmosis in an immunocompetent host from a nonendemic area. Indian J Dermatol. 2016;61(4):467.
57. Wheat LJ et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the infectious diseases society of America. Clin Infect Dis. 2007;45(7):807-25.
58. Mazi PB et al. Management of histoplasmosis by infectious disease physicians. Open Forum Infect Dis. 2022;9(7):OFAC313.
59. NIH. Histoplasmosis: Adult and Adolescent OIs. Available at: https:// clinicalinfo.hiv.gov/en/guidelines/ hiv-clinical-guidelines-adult-and-
adolescent-opportunistic-infections/ histoplasmosis. Last accessed: 22 December 2024.
60. Wheat J et al. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Med. 1995;98(4):336-42.
61. Bobbili K et al. Comparison of posaconazole and itraconazole for treatment of histoplasmosis. Open Forum Infect Dis. 2016;3(Suppl 1):1646.
62. Sabanci R et al. Cardiac implications of itraconazole therapy in histoplasmosis patients. Cureus. 2024;16(4):e59076.
FOR REPRINT QUERIES PLEASE CONTACT: INFO@EMJREVIEWS.COM
The importance of ensuring that patients at risk for HIV are also appropriately screened and protected against hepatitis B, a persistent gap in pre-exposure prophylaxis (PreP) care, is highlighted in this study. The authors compared two PrEP cohorts at a large urban academic center for more than 8 years and revealed declining hepatitis B screening and vaccination rates despite clear national guidance. Their findings shine a spotlight on missed opportunities at the time of prescribing, and signal the need for system-level interventions to educate and optimize preventive infectious disease care.
Shira Doron Tufts Medical Center, Boston, Massachusetts, USA
Authors:


Reham Awad,1 *Melissa E. Badowski1
1. Retzky College of Pharmacy, University of Illinois Chicago, USA *Correspondence to badowski@uic.edu
Disclosure: The authors have declared no conflicts of interest.
Acknowledgements: This research was conducted in full compliance with established ethical principles and applicable regulatory standards, including the Declaration of Helsinki, the Belmont Report, and relevant institutional and federal guidelines. The rights, dignity, and welfare of all participants are of the highest priority. Confidentiality and privacy were strictly maintained. Data was de-identified, securely stored, and accessible only to authorized members of the research team. The research design was developed to minimize potential risks and maximize potential benefits, with careful consideration of vulnerable populations. Any risks to participants will be proportionate to the anticipated benefits and clearly communicated. This study was reviewed and approved by the appropriate Institutional Review Board (IRB) prior to initiation, and all research activities adhered to the approved protocol.
Received: 09.12.25
Accepted: 11.13.25
Keywords: Hepatitis B virus (HBV), HIV, oral pre-exposure prophylaxis (PrEP).
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:76-84. https://doi.org/10.33590/microbiolinfectdisam/MJAT9697
Abstract
Background: HIV pre-exposure prophylaxis (PrEP) with daily oral emtricitabine/tenofovir disoproxil fumarate or emtricitabine/tenofovir alafenamide has been found to be safe and effective in substantially reducing HIV acquisition in high-risk persons. The US Public Health Service PrEP guideline offers recommendations for the screening and vaccination for hepatitis B virus (HBV) in patients who are prescribed oral PrEP. The goal of this study was to compare guideline adherence to the rate of HBV screening and vaccination.
Methods: This study was a retrospective comparative study evaluating the rate of HBV screening and vaccination in patients ≥18 years old and received a prescription for oral PrEP at the University of Illinois Hospital and Health Sciences System (UI Health), Chicago, USA, during two time periods, July 1, 2014–September 30, 2018 (Cohort 1) and October 1, 2018–October 1, 2022 (Cohort 2). Patients currently receiving HBV treatment or with a positive HIV immunoassay blood test at baseline screening were excluded. The primary outcome was comparing appropriate screening and vaccination rates for HBV according to the US Public Health Service PrEP guideline and the CDC, respectively.
Results: A total of 145 patients were included in Cohort 1. The data for these patients were collected through a previous, unpublished study conducted at UI Health and served as historical control data. Of the 230 patients screened for Cohort 2, 145 were included. HBV serology testing prior to PrEP initiation occurred in 78.6% of patients in Cohort 1 compared to 67.6% of patients in Cohort 2, which was statistically significant (p=0.034). HBV vaccine series were initiated by or at first follow-up in 37.9% of patients in Cohort 1 compared to 21.8% of patients in Cohort 2 (p=0.035).
Conclusion: Among patients at UI Health, there was a decrease in HBV screening and vaccination rates in patients within Cohort 2 compared to Cohort 1. There was also a statistically significant difference in human papillomavirus vaccination between both cohorts. Overall, there is an increased need for education among providers prescribing oral PrEP.
1. Appropriate screening for hepatitis B virus (HBV) when prescribing oral HIV pre-exposure prophylaxis (PrEP) remains suboptimal despite oral PrEP receiving approval more than 10 years ago.
2. Upon HBV screening, vaccination rates against HBV continue to remain low.
3. All individuals initiating oral PrEP should receive HBV screening prior to initiation, and HBV vaccination should be offered to those without immunity to HBV.
HIV pre-exposure prophylaxis (PrEP) with oral daily emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF) or FTC/tenofovir alafenamide (TAF) has been proven to be both safe and effective in reducing HIV acquisition when taken as prescribed.1 Prior to medication management, various strategies have been implemented to assist
with the prevention of HIV transmission, including condom distribution, education campaigns, and needle-exchange programs.2-6 Despite these interventions, global HIV transmission continues to occur at alarming rates. FTC/TDF was the first approved HIV prevention option in 2012, used in combination with safe sex practices to reduce HIV-1 transmission. In 2019, another daily oral option for HIV prevention
was approved, FTC/TAF, for men and transgender women who are sexually active.
Despite these significant advances in HIV treatment and prevention measures, in 2022, the CDC reported an estimated 31,800 new HIV infections in the United States.7 However, of the 1.2 million people in the United States currently living with HIV, only one in eight people are aware of their HIV status.8 Furthermore, of those with an indication to receive PrEP, only about 36% were prescribed it in 2022.9 This leads to many individuals having the ability to acquire and transmit the virus. In 2021, the US Public Health Service published an update of the PrEP guideline (“Pre-Exposure Prophylaxis for the Prevention of HIV Infection in the United States: A Clinical Practice Guideline”).1 This guideline offered recommendations for the screening of hepatitis B virus (HBV) in patients prescribed oral PrEP and has been consistent since the guideline was initially published in 2014. The Advisory Committee on Immunization Practices (ACIP) also recommends vaccination against HBV for all individuals based on its 2022 update.10 The CDC reported approximately 14,000 new HBV diagnoses in 2022.11 There is minimal literature published evaluating guideline adherence to HBV serology testing and documented vaccination adherence in people who are prescribed PrEP. Studies in 2013 and 2017 revealed that prescribers screened for HBV in 61% and 38% of people who were initiated on PrEP, respectively.12,13 Furthermore, in 2015, 4,459 individuals were screened for the iPrEx trial, where only 12% were immune to HBV after vaccination.14
The rationale for providing HBV screening and vaccination recommendations for individuals receiving oral PrEP is twofold.1 The first is to decrease HBV transmission, as risk factors for HBV and HIV transmission remain similar. The second is to ensure proper treatment and monitoring of individuals with HBV infection, given that FTC/TDF and FTC/TAF are approved therapies for HBV, and sudden discontinuation may result in an acute flare of HBV. Furthermore, data
highlighting guideline adherence to screening and vaccination of HBV in people prescribed PrEP can serve as guidance to missed opportunities for screening and vaccination administration. This study aimed to assess whether PrEP prescribers at an urban medical center appropriately screened and recommended vaccination administration to people with HBV when initiating oral PrEP.
This single-center, retrospective observational study was conducted at the University of Illinois Hospital and Health Sciences System (UI Health), a 455-bed tertiary care academic medical center with 26 outpatient clinics located in Chicago, USA. The study received approval from the Office for the Protection of Research Subjects Institutional Review Board, with a waiver of informed consent granted. Adults aged 18 years and older who were prescribed oral PrEP (FTC/TDF or FTC/TAF) at a UI Health outpatient clinic between July 1, 2014–September 30, 2018 (Group 1) and October 1, 2018–October 1, 2022 (Group 2) were considered eligible. People were excluded if they were found to be positive for HIV at baseline screening, receiving treatment for chronic HBV, pregnant, or incarcerated. Missing HBV serologies were assumed to lack the primary objective of interest. Lack of documented serology or vaccination status in the encounter note or laboratory data was coded as no screening or vaccination.
This study evaluated data collected from the two time periods. The period between 2014–2018 served as Group 1, while the period between 2018–2022 served as Group 2. These periods of time were based on when the authors’ institutional protocol on oral PrEP was updated. The major difference in PrEP protocols during the two periods of the study centered around expanded indications for prescribing PrEP, along with institutional
education initiatives. Individuals included in this analysis were identified by prescription utilization data.
Data were collected through a retrospective chart review of the electronic medical record (EMR) system at UI Health. Baseline demographic and clinical characteristics, including age, gender identity, race, ethnicity, sexual orientation, weight, height, serum creatinine, and creatinine clearance, were extracted upon initiation of PrEP. Immunization data were obtained from the EMR and Illinois Comprehensive Automated Immunization Registry Exchange (ICARE). For HBV screening and vaccination, the clinic visit date, medication, HIV serology, HBV serology, and documented vaccination history were recorded. To assess adherence to the US Public Health Service guideline and ACIP recommendations published by the CDC, the date that the serologies and documented vaccinations were obtained was recorded.1,10
The primary objective was to determine whether PrEP prescribers at UI Health appropriately screened and recommended vaccination administration for HBV in people initiating PrEP for HIV according to the CDC US Public Health Service guidelines and ACIP recommendations, respectively, since oral PrEP contains agents with activity against HBV. Appropriate screening was defined as HBV serology screening performed at the initial PrEP visit or prior to PrEP initiation. Appropriate vaccination administration was defined as the initiation of the HBV vaccine series by or at the first follow-up visit if HBV serology screening demonstrated a lack of immunity. Secondary outcomes included documentation of immunity to hepatitis A virus (HAV) and HAV vaccination, as well as receipt of human papillomavirus (HPV) and meningococcal vaccinations. These secondary outcomes were included since these infections share common risk factors and transmission routes with HIV.
According to prior data, a 75% incidence of appropriate screening for HBV in Group 1 and a 90% incidence of appropriate screening for HBV in Group 2 were used to establish the effect size. With an α set at 0.05, a sample size of 200 patients was required to achieve 80% statistical power. Data are presented as numbers (percentage) or median [interquartile range], as appropriate. Categorical variables were analyzed using the Chi-squared test or Fisher’s exact test, depending on suitability. Statistical significance was defined as a two-tailed p-value ≤0.05.
Screening was conducted for 397 individuals, and 290 were included in the analysis (Figure 1). Although people could have met multiple exclusion criteria, the most common reason for exclusion was PrEP not prescribed by a UI Health provider (N=51) and a positive HIV immunoassay at baseline (N=30). Of the 290 people included in the final analysis, 145 were included in Group 1 and 145 were included in Group 2. Demographic information and baseline characteristics are summarized in Table 1
Regarding the primary outcome, prior to PrEP initiation, HBV serology testing was performed in 78.6% of participants in Group 1 versus 67.6% in Group 2 (p=0.034; Table 2). Within the population of screened individuals in Group 1, 40% (58/145) were non-immune to HBV, but only 37.9% (22/58; p=0.035) of these people were initiated on the HBV vaccination series by or at the first followup visit. Similarly, within the population of individuals screened in Group 2, 60% (87/145) were found to be non-immune to HBV, but only 21.8% (19/87; p=0.035) of these people started the HBV vaccination series by or at the first follow-up visit. Furthermore, 21.4% (31/145) and 32.4% (47/145) of people were not screened for HBV prior to PrEP initiation at baseline in Groups 1 and 2, respectively (p=0.034). However, 74.2% (23/31) and
397 Patients Screened
Group 1 N=145
Group 2 N=145
107 Patients Excluded
51 not prescribed PrEP by Ul Health provider
30 positive on HIV screening test
22 on post-exposure prophylaxis
1 on HBV treatment
2 pregnant
1 incarcerated
HBV: hepatitis B virus; PrEP: pre-exposure prophylaxis; UI Health: University of Illinois Hospital and Health Sciences System.
61.7% (29/47) of people in Groups 1 and 2, respectively, had no prior HBV immunity or vaccination history documented in the EMR or ICARE (p=0.252). Secondary outcomes included a comparison of vaccination efforts between the groups. No differences existed in the documentation of HAV immunity or vaccination between the groups (p=0.099) or meningococcal vaccination (p=0.332). However, documentation of HPV vaccination was 45% in Group 2 compared with 19% in Group 1 (p<0.00001).
This study demonstrated that prescribers of oral PrEP in outpatient clinics have not increased their efforts to adhere to screening for HBV prior to PrEP initiation over time. Despite the overall decrease, the rate of HBV serology screening at UI Health remains higher than that in the currently available literature (38.3–61.0%).12,13 Of the people who were found to be non-immune to HBV through screening, less than 40% initiated the vaccination series by or at the first follow-up visit. Additionally, more people
were screened for HBV and initiated on the vaccination series in Group 1 than in Group 2, demonstrating that prescribers within the family medicine and infectious diseases clinics, as expected, were more aware of guideline recommendations prior to PrEP initiation than providers in other clinical areas, including internal medicine and primary care clinics. This demonstrates a great need for prescribers outside of infectious diseases care to become more familiar with oral PrEP prescribing practices, since these agents are more likely to be covered by prescription insurance. Regardless of the number of individuals screened and vaccinated in both groups, similar to other published studies, there remained a lack of PrEP prescriptions in communities of color, where the incidence of HIV is highest.15 Black and African American individuals represented 40% of estimated HIV infections, yet fewer than 30% received PrEP prescriptions. Similarly, in 2022, only 15% of women eligible for PrEP were prescribed the medication; this is reflected in the study, where most individuals prescribed PrEP were male.16 Overall, PrEP should be offered to everyone regardless of clinical setting, encompassing sexually active individuals
Table 1: Demographics and baseline characteristics.
CrCL, mL/min
CrCL ≥90
CrCL 60–89
CrCL 30–59
UI Health Outpatient Clinic
Data presented as n (%) or median [IQR].
CrCL: creatinine clearance; IQR: interquartile range; UI Health: University of Illinois Hospital and Health Sciences System.
who do not disclose behaviors associated with HIV acquisition.1 It is especially important to offer PrEP to men of color, since they carry the highest potential for acquiring HIV based on the reported incidence of HIV. In addition to offering recommendations for the screening and vaccination administration of HBV, the guideline also offers recommendations for HIV screening, renal function tests, and hepatitis C screening for patients initiated on oral PrEP. Unlike oral PrEP options, long-acting cabotegravir and lenacapavir for HIV prevention do not require HBV screening prior to initiation.17-19
In addition to the HBV vaccination administration, it is recommended that people receive HAV, HPV, and meningococcal vaccinations. This study demonstrated that there was no difference in the documentation of HAV immunity or vaccination and meningococcal vaccination. However, the overall percentage of individuals with documented vaccinations in both groups was less than 52%. It is important for people who are on PrEP for HIV prevention to understand the risks of acquiring other infections. Persons with HIV (PWH) are at an increased risk of HAV infection, which can compromise
outcome
*N represents patients not immune to HBV.
HAV: hepatitis A virus; HBV: hepatitis B virus; HPV: human papillomavirus; PrEP: pre-exposure prophylaxis.
immune defenses.20 HAV viremia tends to be higher with a prolonged duration when PWH are co-infected. The ACIP recommends routine hepatitis A vaccination for all individuals with HIV aged 1 year and older; however, vaccination rates are less than 25%. Similarly, PWH have an 11- to 24-fold increased risk for meningococcal disease due to a low cluster of differentiation (CD)4 count or high viral load.21 A two-dose vaccine series of meningococcal ACWY (MenACWY) for PWH is recommended by ACIP; however, coverage remains as low as 16.3%. The low vaccination administration rates may be due to a lack of recommendations from providers, a fear of adverse effects, and a lack of expected effectiveness. Conversely, documentation of HPV vaccination administration was significantly greater in Group 2 compared to Group 1. It is important to note that guidance on HPV vaccination recommendations changed in June 2019.22 Before June 2019, the ACIP routinely recommended HPV vaccination administration for those aged 11–12 years,
with catch-up vaccination advised since 2006 for females up to 26 years old and since 2011 for males up to 21 years old. In June 2019, the ACIP acknowledged that certain individuals aged 27–45 years who were not adequately vaccinated could be at risk for new HPV infections and might benefit from vaccination administration.
The limitations of this study included those inherent to its retrospective, single-center design. Prescriber variability likely impacted the results because Group 1 included only people prescribed PrEP in family medicine or infectious diseases clinics. Prescribers who are less likely to prescribe PrEP because of the patient population they serve, such as those in internal medicine and primary care clinics, may not be aware of the U.S. guidance recommendations prior to prescribing PrEP. Additionally, the COVID-19 pandemic, a public health emergency, occurred during Group 2. This may have limited access to provider visits, lab draws, and vaccination
which is why more
in Group 1 appeared to be adherent to the guidance compared to Group 2. Additionally, because this was a retrospective review, serology or vaccination data were considered incomplete if documentation was missing in the medical record.
The findings of this study underscore the critical importance of adhering to guidelines for HBV screening, as this step is frequently overlooked during the prescription of oral PrEP. Additionally, other options for PrEP may be considered, such as cabotegravir or lenacapavir, as they do not have the same screening requirements for HBV prior to initiation, although screening for HBV is recommended at least once in a person’s lifetime, at minimum. Data derived from this study also guided the need for educational efforts. Areas of future research regarding PrEP care include assessing patient barriers, reviewing vaccination administration refusals and why, as well as evaluating prescriber adherence to the other guideline recommendations for patients starting PrEP, including HIV screening, sexually transmitted infection screening, renal function monitoring, and hepatitis C screening.
References
1. Centers for Disease Control and Prevention (CDC): US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 Update: a clinical practice guideline. 2021. Available at: https:// stacks.cdc.gov/view/cdc/112360. Last accessed: September 12, 2025.
2. Rodger AJ et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIVpositive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171-81.
3. Cohen MS et al. Prevention of HIV1 infection with early antiretroviral therapy. New Engl J Med. 2011;365(6):493-505.
4. Chang LW et al. Combination implementation for HIV prevention: moving from clinical trial evidence to population-level effects. Lancet Infect Dis. 2013; 13(1):65-76.
Despite educational efforts on national and global scales to increase PrEP uptake and prescriber knowledge over time, further efforts are necessary to enhance prescriber adherence to HBV screening and vaccination administration guidelines among patients receiving oral PrEP. A decrease in HBV screening and vaccination administration was observed in Group 2 versus Group 1. The results of this study have the potential to guide other clinics in their efforts to provide optimum PrEP and sexual health care. Interventions that could be implemented to improve appropriate guideline adherence to HBV screening and vaccination administration include the creation of an order, set within the EMR when oral PrEP is ordered, to protocolize PrEP initiation and followup; targeted educational initiatives; the creation of pharmacist-led PrEP stewardship programs; or collaborative practice programs involving clinical pharmacists.
5. O'Connor EA et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161(12):874-83.
6. Sherin K et al. What is new in HIV infection? Am Fam Physician. 2014;89(4):265-72.
7. HIV.gov. U.S. statistics. 2025. Available at: https://www.hiv.gov/hiv-basics/ overview/data-and-trends/statistics. Last accessed: 8 July 2025.
8. Centers for Disease Control and Prevention (CDC). HIV surveillance supplemental report: monitoring selected national HIV prevention and care objectives by using HIV surveillance data United States and 6 territories and freely associated states, 2022. 2024. Available at: https:// stacks.cdc.gov/view/cdc/156511. Last accessed: September 12, 2025.
9. Centers for Disease Control and Prevention (CDC). Expanding PrEP
coverage in the United States to achieve EHE goal. 2023. Available at: https://www.cdc.gov/nchhstp/directorletters/expanding-prep-coverage.html. Last accessed: 8 July 2025.
10. Weng MK et al. Universal hepatitis B vaccination in adults aged 19–59 years: updated recommendations of the advisory committee on immunization practices — United States, 2022. MMWR Morb Mortal Wkly Rep 2022;71(13):477-83.
11. Centers for Disease Control and Prevention (CDC). Clinical overview of hepatitis B. 2025. Available at: https:// www.cdc.gov/hepatitis-b/hcp/clinicaloverview/index.html. Last accessed: 8 July 2025.
12. Tellalian D et al. Pre-exposure prophylaxis (PrEP) for HIV infection: results of a survey of HIV healthcare providers evaluating their knowledge, attitudes, and prescribing practices. AIDS Patient Care STDS. 2013; 27(10):553-9.
13. Huang YLA et al. Laboratory testing of a cohort of commercially insured users of HIV preexposure prophylaxis in the United States, 2011–2015. J Infect Dis. 2018;217(4):617-21.
14. Solomon MM et al. The safety of tenofovir–emtricitabine for HIV pre-exposure prophylaxis (PrEP) in individuals with active hepatitis B. J Acquir Immune Defic Syndr. 2016;71(3):281-6.
15. Centers for Disease Control and Prevention (CDC). Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2021. 2023. Available at: http:// www.cdc.gov/hiv/library/reports/ hiv-surveillance.html. Last accessed February 20, 2025.
16. Centers for Disease Control and Prevention (CDC). HIV surveillance supplemental report: monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2019; vol. 26. No. 2. Available at: https://stacks.cdc. gov/view/cdc/160192. Last accessed: September 12, 2025.
17. Dore GJ et al. Frequent hepatitis B virus rebound among HIV- hepatitis B virus-coinfected patients following antiretroviral therapy interruption. AIDS. 2010;24(6):857-65.
18. Marciano S, Gadano A. Why not to stop antiviral treatment in patients with chronic hepatitis B. Liver Int. 2018;38(Suppl 1):97-101.
19. Centers for Disease Control and Prevention (CDC). Screening and testing recommendations for chronic hepatitis
B virus infection (HBV). 2023. Available at: https://www.cdc.gov/hepatitis/hbv/ testingchronic.html. Last accessed: January 4, 2024.
20. Nelson NP et al. Prevention of hepatitis A virus infection in the United States: recommendations of the advisory committee on immunization practices, 2020. MMWR Recomm Rep. 2020;69(5):1-38.
21. Rubis AB et al. Notes from the field: increase in meningococcal disease among persons with HIV – United States, 2022. MMWR Morb Mortal Wkly Rep. 2023;72(24):663-4.
22. Meites E et al. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68(32):698-702.
Author: *Galo Guillermo Farfán-Cano1,2
1. Rey Juan Carlos University, Móstoles, Spain
2. Society of Infectious Diseases of Guayas, Guayaquil, Ecuador
*Correspondence to dr.galo.farfan.cano@gmail.com
Disclosure: The author has declared no conflicts of interest. Written informed consent was obtained from the patient for the publication of this case report, including all clinical details, laboratory results, and anonymized identifiers.
Received: 04.11.25
Accepted: 09.25.25
Keywords: Antiretroviral therapy (ART), cluster of differentiation (CD)4 count variability, diagnostic quality control, global health disparities, HIV management, immunological monitoring, laboratory reliability, resource-limited settings, viral load (VL) testing, WHO.
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:85-91. https://doi.org/10.33590/microbiolinfectdisam/XERL7547
Abstract
Introduction: HIV monitoring in resource-limited settings is often hindered by diagnostic barriers that compromise patient outcomes. Although viral load testing is the gold standard, cluster of differentiation (CD)4 count monitoring remains common due to cost and accessibility limitations.
Case Presentation: The author presents the case of a 38-year-old male in Ecuador who is HIV-positive and undergoing long-term antiretroviral therapy. Despite sustained high adherence, the patient exhibited marked CD4 count variability. A sharp decline 23 months after initial diagnosis (Month 23), followed by a recovery 2 months later (Month 25), raised concerns over laboratory discrepancies and transient clinical conditions.
Discussion: The case highlights the multifactorial nature of CD4 variability, encompassing laboratory quality control, medication supply chains, and biological stressors. A review of the literature supports the role of systemic challenges in such variability, especially in low-resource settings.
Conclusion: Reliable immunological monitoring requires stringent diagnostic protocols, robust healthcare integration, and attention to clinical context, even in patients with stable antiretroviral therapy adherence.
1. Laboratory quality control, regular calibration, and oversight of equipment are crucial to ensure reliable CD4 and viral load testing and prevent errors affecting patient outcomes.
2. A unified global public health network is required to standardize diagnostics, reduce disparities, and foster collaboration between developed and developing countries.
3. Expanding insurance coverage for routine and external diagnostics, integrating specialized testing into social security coverage, and prioritizing comprehensive management of common illnesses in immunocompromised patients can improve holistic care and address systemic inefficiencies.
The global management of HIV continues to face significant challenges, particularly in low- and middle-income countries, where diagnostic capacity and health system infrastructure often limit the effectiveness of treatment monitoring strategies.1-4 Although viral load (VL) testing is recognized as the gold standard for evaluating antiretroviral therapy (ART) efficacy and detecting treatment failure, its routine use is frequently hindered by cost, technological constraints, and limited accessibility in resource-limited settings.5-8 In such contexts, cluster of differentiation (CD)4 count monitoring remains widely utilized despite its lower specificity, providing a more accessible, albeit indirect, measure of immune system status and disease progression.9-12
However, CD4-based monitoring presents its own limitations. Variability in CD4 values can result from pre-analytical and analytical inconsistencies, biological fluctuations, or comorbid conditions, complicating clinical interpretation.9,13,14
This case report presents the clinical course of a 38-year-old male in Ecuador who is HIV-positive and undergoing long-term ART. Despite high adherence and virological suppression, the patient experienced a marked, transient decline in CD4 count followed by spontaneous recovery. The episode raised concerns regarding laboratory accuracy, the impact of minor clinical events,
and potential implications of medication formulation changes.3
By examining this case in the broader context of HIV care in resource-limited settings, the author aims to illustrate the multifactorial nature of CD4 variability and highlight the need for integrated diagnostic strategies, rigorous laboratory oversight, and coordinated public health systems. This report also contributes to the literature by discussing how individual patient outcomes intersect with systemic challenges in monitoring HIV, particularly in settings where VL testing remains inaccessible or delayed.
A 38-year-old male healthcare professional from Ecuador was diagnosed with HIV after presenting for routine testing. At diagnosis, his CD4 count was 167 cells/μL and VL measured 105,156 copies/mL. He reported no prior opportunistic infections and had no history of intravenous drug use or highrisk sexual behavior beyond unprotected intercourse with multiple partners.
Shortly after diagnosis, the patient was initiated on a first-line ART regimen consisting of tenofovir disoproxil fumarate, lamivudine, and dolutegravir.
The combination was initially dispensed by the Instituto Ecuatoriano de Seguridad Social (IESS) through a generic formulation manufactured by Mylan (acquired by Viatris, Canonsburg, Pennsylvania, USA). In mid2024, a change in procurement policy led to a non-clinically justified switch to a formulation by Hetero (Hyderabad, India), also supplied via the Global Fund (Geneva, Switzerland). No adverse effects or clinical deterioration were noted following the change.
The patient reported excellent adherence throughout the treatment period, missing no more than one monthly dose. He also reported concurrent antidepressant therapy (sertraline), as well as a medical history of allergic rhinitis, irritable bowel syndrome, and central abdominal obesity.
During routine follow-up, the patient’s immunological and virological parameters were periodically assessed. Table 1 summarizes the progression of CD4 counts and VL measurements from initial diagnosis to Month 25.
After an expected increase in CD4 count following ART initiation, the patient experienced a marked drop in Month 23 (406 cells/μL), despite maintaining full clinical stability and virological suppression. Two months later, in Month 25, his CD4 count rebounded significantly (759 cells/μL), although VL showed a marginally detectable level (<40 copies/mL).
This sequence raised concerns about the reliability of laboratory results. Notably, the sample from Month 23 was processed in a tertiary-level public hospital, while the sample from Month 25 was analyzed in a private laboratory. The divergence suggested possible analytical variation, technical inconsistencies, or the influence of intercurrent clinical conditions.
The patient denied any major symptoms prior to the Month 23 measurement. However, early in Month 25, he reported a brief episode of low-grade fever and a mild contusion to the lower limb, both of which resolved spontaneously. No ART interruptions or other clinical events were documented during the observed period.

A general upward trend is highlighted, interrupted by the notable decline in Month 23 and subsequent recovery in Month 25. This visual representation emphasizes the transient nature of the immunological fluctuation. CD: cluster of differentiation.
The patient expressed concern regarding the inconsistency in laboratory results and the lack of immediate clinical explanation. He emphasized the need for greater transparency in laboratory practices and for patient-centered communication, especially when unexpected changes in key health indicators arise.
This case illustrates the diagnostic and interpretive complexities involved in HIV monitoring within resource-limited settings, even in the context of excellent ART adherence and apparent clinical stability.
The observed CD4 count fluctuation, particularly the marked decline in Month 23 followed by rapid recovery in Month 25 (Figure 1), raises critical considerations regarding laboratory reliability, biological variability, and systemic health system challenges.5
One of the most salient issues in this case relates to laboratory inconsistencies. As highlighted in previous studies, variability in CD4 count results across laboratories is a well-documented phenomenon, particularly in settings where quality control standards are unevenly implemented.9,13,14
The use of different testing centers (one public and one private) may have introduced pre-analytical or analytical discrepancies, such as differences in sample handling, instrument calibration, or procedural protocols. These concerns are supported by literature emphasizing the central role of rigorous quality assurance in both CD4 and VL monitoring, particularly in decentralized health systems.1,5,6,13
Beyond technical factors, transient clinical conditions can exert a measurable impact on immune markers. Although the patient was asymptomatic at the time of the test in Month 23, he reported a minor febrile illness and physical trauma early in Month 25. These events, albeit clinically insignificant, could have contributed to immune activation and short-term changes in CD4 distribution or turnover.15,16 The influence of low-grade infections and physical or psychological stress on CD4 counts has been previously documented, and must be considered in interpreting unexpected laboratory results.16,17
The patient’s underlying conditions, including abdominal obesity and the use of antidepressants, may also exert a subtle immunomodulatory effect over time. Weight gain associated with ART initiation has been linked to alterations in immune recovery trajectories,17,18 while emerging data suggest that chronic stress and psychiatric comorbidities may influence HIV progression and immune markers.19,20
Although the patient’s ART regimen remained pharmacologically consistent, the non-clinically indicated switch between manufacturers (from Mylan to Hetero) introduces an additional variable.6 Although both formulations are WHO-prequalified generics, evidence from pharmacovigilance studies suggests that post-market variability can exist between batches or manufacturers.6 In this case, the marginally detectable VL
in December (<40 copies/mL) may reflect either a laboratory artifact or a transient viral “blip,” a phenomenon not uncommon even in patients with stable adherence.7,8 Nonetheless, this event underscores the importance of monitoring formulation changes and establishing clear clinical protocols to evaluate potential pharmacological impact.
The broader structural barriers present in many low- and middle-income countries, including delayed access to confirmatory testing, reliance on external laboratories, and fragmented procurement policies, contribute to diagnostic uncertainty and delays in clinical decision-making.21-23 As shown in this case, laboratory inconsistencies are not merely technical but systemic, reflecting gaps in national quality assurance programs and health policy integration.
This case reinforces the urgent need for harmonized diagnostic standards across all levels of care, integration of external laboratory services within a unified health information system, and expansion of insurance coverage for advanced diagnostics in public sector networks.
Compared to previous reports, this case adds nuanced insights into the interaction between laboratory quality, minor clinical events, and health system structures in shaping the interpretation of CD4 variability. While prior studies have addressed these dimensions in isolation,5-8,13,16 few case-based analyses have illustrated their convergence in a real-world clinical scenario from Latin America.
The report underscores the limitations of relying exclusively on immunological parameters when VL testing is inconsistently accessible, as is the case in many parts of the Global South. In this context, patientcentered clinical judgment, supported by system-level reforms in diagnostic
integration, remains critical to ensure accurate and timely decisions in HIV care.
This case highlights the complex and interdependent challenges inherent to HIV monitoring in resource-limited settings, where clinical decision-making is often determined as much by systemic constraints as by clinical data. In Ecuador, as in many low- and middle-income countries, essential diagnostic tools such as HIV drug-resistance genotyping, advanced immunological assays, and comprehensive sexually transmitted infection screening remain outside the scope of national reimbursement schemes, despite being considered standard of care under international guidelines. The prohibitive cost of resistance testing, frequently exceeding 2,000 USD per patient, renders such analyses inaccessible to most individuals and healthcare institutions.
As a consequence, national HIV programs are forced to rely on a uniform, first-line, ART initiation policy, with regimen modifications occurring only after immunological or virological failure is detected, rather than being guided by proactive resistance profiling.
References
1. Greig J. et al. Viral load testing in a resource‐limited setting: quality control is critical. J Int AIDS Soc. 2011;14:23.
2. Adebamowo CA et al. Challenges in the detection, prevention, and treatment of HIV-associated malignancies in low- and middle-income countries in Africa. J Acquir Immune Defic Syndr. 2014;67(Suppl 1):S17-26.
3. Bekker LG et al. Advancing global health and strengthening the HIV response in the era of the Sustainable Development Goals: the International AIDS Society-Lancet Commission. Lancet. 2018;392(10144):312-58.
4. Sester M et al.; European Network for global cooperation in the field of AIDS and TB (EUCO-Net). Challenges and perspectives for improved management
This reactive model not only delays optimal clinical management but also increases the likelihood of misinterpreting transient laboratory fluctuations, such as CD4 variability, as indicators of treatment failure. The burden of diagnostic uncertainty thereby shifts from institutional systems to individual clinicians and patients.
This report underscores that CD4 variability must be interpreted within a multidimensional framework encompassing analytical reliability, biological fluctuation, and systemic limitations. Even with strict ART adherence and clinical stability, diagnostic inconsistency can obscure treatment evaluation and undermine trust in laboratory monitoring.
Ultimately, the findings presented here exemplify the broader structural inequities that shape HIV care globally. The inability to fully implement evidence-based diagnostic protocols in under-resourced health systems demands urgent policy reform. Guaranteeing access to high-quality, standardized, and cost-effective diagnostic testing, particularly for VL and resistance genotyping, should no longer be viewed as an aspirational goal but as a fundamental prerequisite for equitable, effective, and sustainable HIV care.
of HIV/ Mycobacterium tuberculosis coinfection. Eur Respir J. 2010;36(6):1242-7.
5. MSF - Médecins Sans Frontières. Undetectable - how viral load monitoring can improve HIV treatment in developing countries. 2012. Available at: https:// www.msf.org/sites/default/files/2018-08/ undetectable-how-viral-load-monitoringcan-improve-hiv-treatment-indeveloping-countries.pdf. Last accessed: February 2025.
6. Roberts T et al. Scale-up of routine viral load testing in resource-poor settings: current and future implementation challenges: current and future implementation challenges. Clin Infect Dis. 2016;62(8):1043-8.
7. Roberts T et al. Challenges and opportunities for the implementation of virological testing in resource‐
limited settings. J Int AIDS Soc. 2012;15(2):17324.
8. Kippen A et al. The viral load monitoring cascade in HIV treatment programmes in sub-Saharan Africa: a systematic review. BMC Public Health. 2024;24(1):2603.
9. Organización Panamericana de la Salud (OPS). Plan estratégico de la Organización Panamericana de la Salud 2020-2025: la equidad, el corazón de la salud. 2020. Available at: https:// www.paho.org/es/documentos/planestrategico-organizacion-panamericanasalud-2020-2025. Last accessed: February 2025.
10. Barnett D et al. CD4 immunophenotyping in HIV infection. Nat Rev Microbiol. 2008;6(Suppl 11):s7-15.
11. Vajpayee M, Mohan T. Current practices in laboratory monitoring of HIV infection.
Indian J Med Res. 2011;134(6):801-22.
12. Pham MD et al. Acceptability and feasibility of point-of-care CD4 testing on HIV continuum of care in low and middle income countries: a systematic review. BMC Health Serv Res. 2016;16(a):343.
13. Ministerio de Sanidad. PROGRAMA DE CONTROL DE CALIDAD DE LA CARGA VIRAL DEL VIH. 2003. Available at: https://www.sanidad.gob.es/ciudadanos/ enfLesiones/enfTransmisibles/sida/ docs/control_calidad.pdf. Last accessed: February 2025.
14. National AIDS Control Organisation (NACO). National guidelines for HIV-1 viral load laboratory testing. 2018. Available at: https://www.naco.gov.in/sites/default/files/ NationalGuidelinesForHIV-1ViralLoadLaboratoryTestingApril2018%20%282%29.pdf.
Last accessed: February 2025.
15. Naif HM. Pathogenesis of HIV infection. Infect Dis Rep. 2013;5(Suppl 1):e6.
16. Duggal S et al. HIV and malnutrition: effects on immune system. Clin Dev Immunol. 2012;2012:784740.
17. Bailin SS et al. Obesity and weight gain in persons with HIV. Curr HIV/AIDS Rep. 2020;17(2):138-50.
18. Chandiwana NC et al. Weight gain after HIV therapy initiation: pathophysiology and implications. J Clin Endocrinol Metab. 2024;109(2):e478-87.
19. Ellis RJ et al. Mechanisms underlying HIV-associated cognitive impairment and emerging therapies for its management. Nat Rev Neurol. 2023;19(11):668-
87. Erratum in: Nat Rev Neurol. 2023;19(12):787.
20. Prakash P et al. HIV-associated hypertension: risks, mechanisms, and knowledge gaps. Circ Res. 2024;134(11):e150-75.
21. Farfán-Cano GG. Emotional and clinical challenges of delayed HIV seroconversion – case study. Futur Med. 2024;3(3):28-35.
22. Bouabida K et al. Challenges and barriers to HIV care engagement and care cascade: viewpoint. Front Reprod Health. 2023;5:1201087.
23. Wise JM et al. Barriers to HIV testing: patient and provider perspectives in the Deep South. AIDS Behav. 2019;23(4):106272.
Authors: *Myra Nasir,1 Samuel Stone,2 Ian Mahoney,3 Justin Chang,4 Julie Kim,5 Sajani Shah,6 Laura A. McDermott,1 Paola Sebastiani,7 Hocine Tighiouart,7,8 David R. Snydman,1 Shira Doron1
1. Department of Medicine, Division of Geographic Medicine and Infectious Diseases, Tufts Medical Center, Tufts University School of Medicine, Boston, Massachusetts, USA
2. Department of Medicine, Division of Cardiology, Tufts Medical Center, Boston, Massachusetts, USA
3. Department of Medicine, Division of Pulmonary Critical Care and Sleep Medicine, New York University, USA
4. Department of Surgery, Division of General Surgery, University of Illinois, Chicago, USA
5. Department of Surgery, Mount Auburn Hospital, Cambridge, Massachusetts, USA
6. Department of Surgery, Division of Bariatric Surgery, Tufts Medical Center, Boston, Massachusetts, USA
7. Department of Medicine, Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Tufts University School of Medicine, Boston, Massachusetts, USA
8. Department of Medicine, Tufts Clinical and Translational Science Institute, Tufts University, Boston, Massachusetts, USA
*Correspondence to myranasir1@gmail.com
Disclosure: Nasir, Stone, Tighiouart, and Doron have received grants from Tufts University Collaborates and NIH CTSA; and non-financial support (study drug) from Martek Biosciences Corporation (manufactured by Chr Hansen, Inc). Sebastiani has declared no conflicts of interest.
Acknowledgements: This study was supported by a grant from Tufts University Collaborates and NIH CTSA grant UM1TR004383 and UM1TR004398 awarded to Shira Doron, Julie Kim, and David R. Snydman.
Received: 12.16.24
Accepted: 11.05.25
Keywords: Bariatric surgery, gastrointestinal microbiome, Lactobacillus rhamnosus GG (LGG), obesity, pilot clinical trial, postoperative weight loss, probiotic colonisation, probiotics, randomized double blind, total weight loss.
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:92-101. https://doi.org/10.33590/microbiolinfectdisam/PAGL4460
Abstract
Introduction and Objectives: There is increasing evidence suggesting the impact of human gut microbiota on digestion and metabolism. It is hypothesized that the microbiome in people who are obese is more efficient than that in people who are lean in absorbing energy from food, thus predisposing them to weight gain. A transformation in gut microbiota has been demonstrated in patients who have undergone bariatric surgery, which has been positively associated with postsurgical weight loss. However, there is a lack of studies investigating the impact of probiotics on weight loss in post-bariatric surgery patients. The authors conducted a pilot clinical trial to investigate the impact of a probiotic, Lactobacillus rhamnosus GG (LGG), on weight loss and quality of life in patients who have undergone bariatric surgery.
Methods: The study was registered with ClinicalTrials.gov NCT01870544. Participants were randomized to receive either LGG or placebo capsules. Percent total weight loss at their postoperative visits was calculated, and differences between the two groups were tested using a t-test with unequal variances. The effect of LGG on Gastrointestinal Quality of Life Index (GIQLI) scores was estimated using a mixed model for repeated measures.
Results: The mean rate of change in percent total weight loss at post-operative visit 1 for the placebo and treatment groups was 0.098 and 0.079 (p=0.41), respectively, whereas that at postoperative visit 2 was 0.148 and 0.126 (p=0.18), respectively. The difference in GIQLI scores on 30-day and 90-day visits was 0.5 (-7.1–8.0), p=0.91, and 3.7 (-4.9–12.3), p=0.42, respectively. LGG was recovered from the stools of three out of five participants in the treatment group.
Conclusion: The authors did not appreciate a significant difference in the mean rate of weight loss or GIQLI scores between the groups that received LGG versus placebo. This study demonstrated the survival of Lactobacillus during transit through the gastrointestinal tract.
1. Bariatric surgery transforms gut microbiota and drives weight loss, but whether probiotics improve outcomes remains uncertain; clinicians ask if Lactobacillus rhamnosus GG (LGG) could enhance postoperative weight or quality of life.
2. This randomized, double-blind, placebo-controlled pilot trial enrolled post-bariatric patients to receive LGG or placebo for 44 days, assessing total weight loss, Gastrointestinal Quality of Life Index (GIQLI) scores, safety, and stool recovery of LGG.
3. LGG did not significantly change weight loss or GIQLI versus placebo; recovery in three of five stools confirms survival. Probiotics to accelerate weight loss appear unwarranted, pending larger, longer-duration studies.
Obesity is a major public health problem worldwide. In the USA, 66% of the population is classified as overweight or obese.1
The ‘microbiome revolution’ has, in the last 20 years, brought our attention to the importance of human gut microbiota as a factor affecting health and disease.2 Gut microbiota include a range of microorganisms that inhabit the gastrointestinal tract and have been implicated in digestion and metabolism, protection from pathogens, insulin resistance, and neurologic functioning.3-7 Metagenomics and twin studies have revealed that certain microbial compositions are associated with lean
body composition, and alteration of these microbial communities is associated with weight gain and metabolic disease.8,9 In people with obesity, the flora of the gut has been found to be less diverse, less rich, and to have an increased ratio of Firmicutes-toBacteroidetes as compared to normal weight individuals.10 It is hypothesized that the obese microbiome is more efficient than the lean microbiome in absorbing energy from food, thus predisposing to weight gain.8
Bariatric surgery is the most effective treatment for the achievement of prolonged weight loss and has been associated with improvement in diabetes and hypertension.11,12 Studies suggest that the beneficial health effects of these surgeries are not only explained by procedure-induced food restriction and malabsorption but also by alterations of neuroendocrine and immune signaling pathways.13 An increase in Bacteroidetes and Proteobacteria with a decrease in Firmicutes after bariatric surgery has been demonstrated. When the Bacteroidetes/Firmicutes ratio after bariatric procedures was used as a surrogate marker for the observed changes in the obese microbiome compared to the lean microbiome, it was positively associated with post-surgical weight loss.14
Lactobacillus rhamnosus GG (LGG) is a widely studied probiotic, especially with regard to its influence on metabolic syndrome. The authors chose the LGG strain because, compared to other strains, it demonstrated the fastest growth while also being stable in stomach acid and bile, capable of surviving the upper gastrointestinal tract to reach the colon, adhering well to intestinal epithelial cells, and producing antimicrobial substances active against various pathogenic and commensal gastrointestinal bacteria.15 A favorable impact on obesity and diabetes has been demonstrated in mice with diet-induced obesity, showing evidence of improvement in insulin resistance with LGG.16,17
Whereas there is increasing evidence to suggest that use of probiotics is linked with
lower inflammatory state and weight loss, there is a lack of studies investigating the impact of probiotics on weight loss in patients who have undergone bariatric surgery.18-20
To inform future studies, the authors conducted a pilot trial to investigate the impact of LGG on weight loss in patients who have undergone bariatric surgery.
This was a randomized, double-blind, placebo-controlled pilot trial to investigate whether weight loss and changes in quality of life differ after bariatric surgery in patients who did and did not receive LGG. The primary outcome is the rate of change of percent total weight loss. Secondary outcomes are the Gastrointestinal Quality of Life Index (GIQLI) on the day of surgery, at post-operative visit 1 and visit 2, adverse event percentages, and recovery of LGG from stool.
Participants were recruited from Tufts Medical Center in Boston, Massachusetts, USA, from June 24, 2013–January 31, 2016, if they were scheduled for elective gastric surgery for weight reduction. Patients were eligible for the study if they were at least 18 years of age, were evaluated for surgery as outpatients, and had stable comorbidities. Exclusion criteria included active colitis; recent or planned receipt of radiation or chemotherapy; being on active immunosuppressive medication; known or suspected allergies to probiotics, lactobacillus, milk protein, or microcrystalline cellulose, or allergy to two or more of the rescue antibiotics that might be used to treat Lactobacillus infection (ampicillin, clindamycin, and moxifloxacin) should that become necessary; and positive baseline stool culture for LGG.
Participants included in the study were restricted from consuming certain yogurt; however, since yogurt is a dietary staple after
bariatric surgery, to improve enrollment after initial slow accrual, the protocol was later amended to allow for types of yogurts that contained select live cultures that would be expected to have minimal effect on patients’ microbiota, as well as yogurt without cultures. Four commercially available yogurts were permitted.
Patients undergoing sleeve gastrectomy were initially planned to be excluded from the study. However, sleeve gastrectomy rapidly became the preferred weight-loss surgery, which resulted in significantly fewer gastric bypass surgeries at xxx Medical Center than at the time of initial study design. Therefore, in order to improve enrollment, the study design was amended to include patients undergoing sleeve gastrectomy, in addition to gastric bypass surgery.
The trial design is parallel with an allocation ratio of 1:1. Sample size analysis was not conducted since this was a pilot study to inform a future larger-scale trial.21
The random allocation was done by the statistician using a random number generator with the allocation sequence provided directly to the research pharmacy, which labeled the pills with the subject names but without identification of the compound. Study staff, which included investigators and research assistants, enrolled participants and went to the research pharmacy to pick up the study compound already labeled for the subject. Thus, investigators, study staff, and participants were blinded, as were the clinical staff caring for these participants.
Participants were randomized to receive either probiotic capsules of LGG (1x1010 organisms per capsule) or identical appearing placebo capsules provided by Martek Biosciences Corporation (Columbia, Maryland, USA), manufactured by Chr Hansen, Inc. (Hørsholm, Denmark), to be taken twice daily for 44 days, beginning 14 days before surgery. Pill bottles contained
100 capsules. Capsules were collected from each subject prior to initiation of the course and at study completion. One capsule from each time period was cultured to confirm the viability of the organism and organism counts. Ten extra capsules were provided in case of loss of capsules. Remaining capsules were counted at the end of the study period to assess adherence. Stool samples were obtained at baseline (2–4 weeks before surgery), on the day of surgery, and at postoperative visit 1 and post-operative visit 2 to assess for the presence of colonization by LGG.
To monitor for adverse events, participants completed questionnaires about the presence or absence of certain symptoms before and after they began taking the study compound. These questionnaires were completed at the first surgeon visit, first pre-operative visit, and day of surgery in order to establish a baseline, then at the first four weekly postoperative telephone check-ins, and postoperative visit 1 and 2.
All participants received routine pre-operative prophylactic antibiotics to prevent surgical site infection. One subject in the placebo group was receiving azithromycin preoperatively for suspected bacterial infection and continued it for several days after surgery.
The study was registered with ClinicalTrials. gov NCT01870544, and was approved by the xxx Medical Center Institutional Review Board. Written informed consent was taken from all participants. CONSORT reporting guidelines were used (Supplement Table 1; Appendix 1).22
Demographic information, history of chronic diseases, and medications were collected at screening. Participants were asked to complete the validated GIQLI questionnaire at the pre-operative visit, on the day of surgery, and at post-operative visit 1 and visit 2.23 Study weight measurements were taken at
63 subjects expressed interest
26 excluded (lost to follow-up/did not meet inclusion criteria)
15 withdrew prior to randomization,
3 withdrew after randomization and before study drug, 1 received study compound and was lost to follow-up
37 subjects consented
10 subjects included in the placebo group
3 subjects underwent RYGB
18 subjects completed study
7 subjects underwent sleeve gastrectomy
the preoperative visit, the day of surgery, and at post-operative visit 1 and visit 2. Weights were also extracted from the electronic medical record when available from visits not part of the study.
8 subjects included in the treatment group
2 subjects underwent RYGB
6 subjects underwent sleeve gastrectomy
Compared to other metrics such as excess weight loss and change in BMI, percent total weight loss (%TWL) has been found to have the least variability when stratified by preoperative patient characteristics, and therefore, is the metric used in this study.24
Subject weights were measured during post-operative visits. Percent total weight loss at X days (%TWL_X) was calculated as (weight at surgery – weight at visit)/weight at surgery x X/(delta day), where delta day was the difference in days between the date of surgery and the date of visit. Differences in %TWL_X between the two groups were tested using a t-test with unequal variances.
Participants were given stool collection kits consisting of a toilet hat, gloves, tongue depressors, specimen cups, and paper bags, and asked to produce and save a stool sample in their freezer within 24 hours of their appointment date and to bring the sample to their scheduled visit. Samples were then aliquoted and frozen at –80 °C.
Stool samples and capsules were serially diluted and plated in duplicate onto Lactobacilli selective agar supplemented with tomato juice and glacial acetic acid. Plates were incubated for 48 hours in an anaerobic chamber (5%CO2, 10%H2, 85% N2) at 35 +/- 2 oC and were then observed for characteristic growth and colonial morphology. Presumptive LGG colonies, appearing as white and creamy with a distinct buttery smell, were enumerated. The palisading appearance of LGG colonies on Gram stain was used to distinguish it from other Lactobacillus species. All strains presumptively identified were run through the APIZYM® (Biomerieux, Marcy-l’Étoile, France), an identification kit that differentiates between the Lactobacillus species on the basis of enzymatic reactions. Isolates that were consistent with LGG by APIZYM® were additionally run through the API CH-50® (Biomerieux) system, which differentiates between different species of Lactobacillus on the basis of carbohydrate fermentation.
The effect of LGG on the continuous GIQLI scores was estimated using a mixed model for repeated measures. The outcome for each subject consisted of all follow-up measurements, including the baseline measurement. All variables included in the model were treated as fixed effects, and included categorical time, randomization, and their interaction. The authors performed sensitivity analyses to account for missing data in follow-up using pattern mixture models (PMM). They used the core functionality available in SAS PROC MI and MIANALYZE procedures to implement two types of PMM: standard PMM and control-based PMM.
A total of 37 bariatric surgery candidates were recruited and followed up through April 2016. Of these, 15 participants withdrew prior to randomization, three after randomization and before study drug was administered, and one received the study drug but was lost to follow-up, with 18 participants completing follow-up (Figure 1). Ten participants received oral placebo, and eight received oral LGG. Table 1 compares the characteristics of participants by study group. The median age of the placebo group and the treatment group was 47.5 and 46.5, respectively. Out of 18 participants, 12 were female, comprising 60% of the placebo group and 75% of the treatment group. Of those who received LGG, compliance was variable: in the treatment group, the median of doses missed was 5.5 (interquartile range: 0–9), and that in the placebo group was 3 (interquartile range: 0–19). Baseline median BMI and excess weight of the placebo group (42.44 and 103.02 lbs, respectively) were comparable to the baseline median BMI and excess weight of the treatment group (41.16 and 102.28 lbs). The placebo group, as compared to the treatment group, had a higher percentage of patients with diabetes (40% versus 25%, respectively) and a lower percentage of
patients with hyperlipidemia (20% versus 25%, respectively). Most participants underwent sleeve gastrectomy (70% and 75% for placebo and treatment groups, respectively) while the rest underwent Rouxen-Y gastric bypass.
At baseline, mean (SD) GIQLI scores for treatment and placebo groups were 59.0 (6.1) and 59.4 (7.2), respectively. On the day of surgery, the GIQLI score was 61.1 (6.3) for the treatment group, as compared to 57.4 (9.2) for the placebo group, with a mean difference (95% CI) of 3.7 (–3.8–11.2), p=0.35. At postoperative visit 1, the GIQLI score was 55.1 (8.5) for the treatment group, as compared to 55.5 (4.0) for the placebo group, with a difference of 0.5 (–7.1–8.0), p=0.91. At postoperative visit 2, the GIQLI score was 60.7 (4.9) for the treatment group, as compared to 56.5 (6.8) for the placebo group, with a difference of 3.7 (–4.9–12.3), p=0.42.
The mean time between surgery and postoperative visit 1 for the treatment and placebo groups was 34 and 54 days, with a median of 38 and 48 days, respectively. The mean time between surgery and postoperative visit 2 for the treatment and placebo groups was 105 and 125 days, with a median of 107 and 111 days, respectively. The mean rate of change in %TWL at post-operative visit 1 for the placebo and treatment groups was 0.098 and 0.079 (p=0.41), respectively, whereas that at the post-operative visit 2 was 0.148 and 0.126 (p=0.18), respectively.
LGG was not recovered from any stool samples from participants belonging to the placebo group. Among participants in the treatment group, no samples were successfully obtained after completion of the course of treatment. All samples were taken during the treatment course. Stool samples were collected from only five of the eight participants in the treatment group after the start of the course of treatment. Of the five, LGG was recovered from the stool samples of three participants. One of the participants from whom LGG was not recovered was later
found to have missed 19 doses, and the other four doses.
The most common adverse events experienced by the study and placebo groups while taking the study compound were increased frequency of bowel movements, loss in appetite, flatulence, constipation, rumbling, looser stools, and abdominal fullness (Table 2).
In the authors’ pilot study of patients who underwent bariatric surgery, they did not appreciate a clinical or statistically significant difference in the mean rate of weight loss between the groups who received LGG versus placebo. Their study findings are consistent with results demonstrated by a systematic review and meta-analysis of five randomized controlled trials, including a total of 279 patients after bariatric surgery, in which no significant difference in percent excess weight loss at 3 months, change in
BMI, waist circumference change, or fat mass change was noted between the probiotic and placebo groups.25 However, it should be noted that if there were some minor impact of oral probiotics on weight loss, this may be masked by the substantial effect of bariatric surgery on weight loss, resulting in no measurable difference between the two groups.
This is the first study, to the authors’ knowledge, to evaluate whether probiotics can be recovered in the stool of patients who have undergone bariatric surgery. To exert its beneficial properties, probiotics must survive during their transit through the low pH environment of the stomach and the lytic enzymes present in the small intestine. In the authors’ study, LGG was recovered from three of the five participants in the treatment group, demonstrating survival, though inconsistent, of the Lactobacillus during transit through the gastrointestinal tract. Several factors may have contributed to the lack of recovery of LGG from two participants in the treatment group who were still in the treatment phase, including adherence, the constituents of the participants’ diet, which can affect survival and growth of the bacteria, the stability of the culture, and the size of the inoculum, which affects the survival of probiotic bacteria.
There was no significant difference in the GIQLI scores within the follow-up period between the study and placebo groups.
In the authors’ pilot study of patients undergoing bariatric surgery, LGG was found to be safe. Adverse event rates were similar in the two groups and consistent with what would be expected in patients who have undergone gastrointestinal surgery.
References
1. Wang Y et al. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity. 2008;16(10):2323-30.
2. Petersen JM, Osvatic J. Microbiomes in natura: importance of invertebrates in understanding the natural variety of animal-microbe interactions. mSystems. 2018;3(2):10-128.
Growing evidence supports that weightloss interventions, whether dietary or surgical, lead to changes in the composition of the gastrointestinal microbiome.26 However, there are conflicting data regarding the impact of probiotics on weight loss. Whereas Kadooka et al.27 demonstrated a significant decrease in weight and body fat in participants who received fermented milk with Lactobacillus compared to a control group. A meta-analysis by Mohammadi et al.28 of nine RCTs, including 410 participants, showed no significant difference in BMI or percentage of excess weightloss between the probiotic and control groups.28
The authors study had several limitations. It was a pilot study and, therefore, not intended to be fully powered to detect statistically significant differences in outcomes between groups. Though the follow-up outpatient visits were intended to be at 1 and 3 months post-surgery, there was significant variability in the number of days after surgery at which participants were evaluated. The 3-month duration of follow-up may not have been long enough to demonstrate a clinically significant difference in weight loss or quality of life scores between the two groups.
Future trials should investigate approaches to enhance LGG colonization, including the use of higher probiotic doses, evaluation of alternative or complementary strains, and optimization of delivery methods such as sustained-release formulations or coadministration with prebiotics to improve bacterial survival and engraftment in the gastrointestinal tract.
3. Rothschild D et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210-5.
4. Mills S et al. Precision nutrition and the microbiome, part I: current state of the science. Nutrients. 2019;11(4):923.
5. Kelly CJ et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17(5):662-71.
6. Zheng P et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317.
7. Kelly JR et al. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
8. Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. Journal of Physiology. 2009;587(17):4153-8.
9. Turnbaugh PJ et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480-4.
10. Ley RE et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070-5.
11. Tadross JA, Le Roux CW. The mechanisms of weight loss after bariatric surgery. Int J Obes. 2009;33(1):S28-32.
12. Federico A et al. Gastrointestinal hormones, intestinal microbiota and metabolic homeostasis in obese patients: Effect of bariatric surgery. In Vivo; 2016;30(3):321-30.
13. Ionut V, Bergman RN. Mechanisms responsible for excess weight loss after bariatric surgery. J Diabetes Sci Technol. 2011;5(5):1263-82.
14. Luijten JC et al. The Importance of the microbiome in bariatric surgery: a systematic review. Obes Surg. 2019;29(7):2338-49.
15. Doron S et al. Lactobacillus GG: bacteriology and clinical applications. Gastroenterol Clin North Am. 2005;34(3):483-98.
16. Ji Y et al. Modulation of active gut microbiota by Lactobacillus rhamnosus GG in a diet induced obesity murine model. Front Microbiol. 2018;9:710.
17. Kim SW et al. Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat dietfed mice through enhancement of adiponectin production. Biochem Biophys Res Commun. 2013;258-63.
18. Hegazy SK, El-Bedewy MM. Effect of probiotics on pro-inflammatory cytokines and NF-κb activation in ulcerative colitis. World J Gastroenterol. 2010;16(33):4145.
19. Ashraf R, Shah NP. Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr. 2014;54(7):93856.
20. Dror T et al. Microbiota manipulation for weight change. Microb Pathog. 2017;106:146-61.
21. Eldridge SM et al.; PAFS consensus group. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355.
22. Schulz KF et al. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Annals of Internal Medicine. 2011;154(4):291-2.
23. Eypasch E et al. Gastrointestinal quality of life index: development, validation and application of a new instrument. Br J Surg. 1995;82(2):216-22.
24. Grover BT. Defining weight loss after bariatric surgery: a call for standardization. Obes Surg. 2019;29(11):3493-9.
25. Daghmouri MA et al. Probiotics in bariatric surgery ensure greater lipids and glycemic profile with no effect on anthropometric measurements and inflammatory markers: a systematic review and meta-analysis of RCT. Surg Open Dig Adv. 2022;7:100061.
26. Seganfredo FB et al. Weight-loss interventions and gut microbiota changes in overweight and obese patients: a systematic review. Obes Rev. 2017;18(8):832-51.
27. Kadooka Y et al. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br J Nutr. 2013;110(9):1696-703.
28. Mohammadi H et al. Effects of pro-/synbiotic supplementation on anthropometric and metabolic indices in overweight or obese children and adolescents: a systematic review and meta-analysis. Complement Ther Med. 2019;44:269-76.
29. Gastrointestinal Surgery for Severe Obesity. NIH Consensus Statement Online 1991 25 27;9(1):1-20.
30. Gorbach SL. The discovery of Lactobacillus GG. Nutrition Today. 1996;31:25-45.
31. Flegal KM et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235-41.
32. Turnbaugh PJ et al. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213-23.
33. Ley RE et al. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444(7122):1022-3.
34. Sharma BK et al. Intragastric bacterial activity and nitrosation before, during, and after treatment with omeprazole. British Medical Journal. 1983;(289): 717-9.
35. Stearns JC et al. Bacterial biogeography of the human digestive tract. Scientific Reports. 2011;1:170.
36. Von Rosenvinge EC et al. Immune staus, antibiotic medication and pH are associated with changes in the stomach fluid microbiota. The ISME Journal. 2013:(7):1354-66. 20.
37. Saxelin M et al. Dose response on the faecal colonisation of Lactobacillus strain GG administered in two different formulations. Microbial Ecology in Health and Disease. 1995;6(3):119-2.
38. Turnbaugh P.J. et al. The human microbiome project. Nature, 2007:449(7164):804-10.
39. Steele P. Consumer insights from the industry perspective: the human microbiome, diet, and health. A food forum workshop. DuPont Nutrition and Health. 2012.
40. Bayer AS et al. Therapy of experimental infective endocarditis due to antibiotictolerant Lactobacillus plantarumbactericidal synergy of penicillin plus gentamicin. Correlation of in vitro susceptibility studies with in vivo efficacy. Chemotherapy. 1981;27(6):44451.
41. Cole JR et al. The ribosomal database project (RDP-II): Introducing myRDP space and quality controlled public data. Nucleic Acids Res. 2007;35:D169-72.
42. Cole JR et al. The ribosomal database project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141-5.
43. Wang Q et al. Naive bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261-7.
44. Good I. The population frequencies of species and the estimation of population parameters. Biometrika. 1953;40:237-64.
45. Begon M, Harper J, Townsend C, eds. Ecology - individuals, populations, communities. 1990. 2nd edition. Oxford: Blackwell Scientific Publications.
46. Chao A. Non-parametric estimation of the number of classes in a population. Scandinavian Journal of Statistics. 1984;11:265-70.
47. Hayek L, MA B, eds. Surveying natural populations. New York City: Columbia University Press; 1996.
48. White JR et al. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. 2009;5(4):e1000352.
49. Schlss P et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537-41.
Authors: *Zolf Ali Dawlatpoor,1 Murtaza Abdullahi,2 Zaman Nowruzi,3 Parwana Nazir Furmoli,4 M. Naser Movahhedi,5 Bahara Sadat,6 Zohra Majidi,3 Moqadasa Mohseni,6 Ahmad Farid Habibyar,7 Nigina Fazali8
1. Ministry of Public Health, Afghanistan Food and Drugs Authority (AFDA), Kabul, Afghanistan
2. Aqa Khan Health Services, Afghanistan (AKHS, A), Kabul, Afghanistan
3. Bayazid Rokhan University, Kabul, Afghanistan
4. Quality Assurance, Afghanpharma Ltd, Kabul, Afghanistan
5. Kateb University, Kabul, Afghanistan
6. Drug Quality Assessment, Particip GMBH, Kabul, Afghanistan
7. Department of Pharmacology, Kabul University, Afghanistan
8. Shinozada Hospital, Kabul, Afghanistan
*Correspondence to zolfalidawlatpoor45@gmail.com
Disclosure:
The authors have declared no conflicts of interest. This study was approved by the Ethics Committee of Kabul University, Faculty of Pharmacy, Afghanistan. Participation was voluntary, and informed consent was obtained from all respondents prior to data collection. The study was conducted in accordance with the Declaration of Helsinki.
Acknowledgements: The authors want to express their high appreciation and deep gratitude to Professor Qand Aqa Nzari, Associate Professor Shafiq Mashal, Associate Professor Raihan, Teacher Sadiah, Professor Bbak, directors, supervisors of pharmacy of Kabul University, data collectors, and respondents from all ten health regions of Kabul University for their cooperation and contribution towards the success of this work.
Received: 09.09.25
Accepted: 11.10.25
Keywords: Antibiotic, attitude, Kabul University, knowledge.
Citation: Microbiol Infect Dis AMJ. 2025;3[1]:102-111. https://doi.org/10.33590/microbiolinfectdisam/WJKD4132
Abstract
This cross-sectional study aimed to assess the knowledge and attitudes of students at Kabul University, Afghanistan, regarding antibiotics and their use. A total of 1,073 validated self-administered questionnaires were distributed at Kabul University. The questionnaire included questions about accessibility, attitudes, effectiveness, and antibiotic resistance. Students' knowledge levels were measured by counting correct answers and categorized into poor, medium, and high. Results showed that 81% of respondents were aware of antibiotics, and 52.1% used antibiotics only with a doctor's prescription, but 77.2% stopped taking antibiotics once they felt better. Only 24% knew that amoxicillin is an antibiotic, and 63% had poor overall knowledge about antibiotics. The study revealed negative attitudes, many misconceptions, and insufficient knowledge among students, highlighting the need for community education campaigns at the start of each university semester to properly inform students about the proper use, effectiveness, and antibiotic resistance issues.
1. In this cross-sectional survey of 1,073 students, 63% had poor knowledge and 77% stopped treatment early, highlighting the need for community education campaigns about the proper use of antibiotics.
2. Validated self-administered questionnaires were disseminated across 22 faculties and revealed that only 24% knew that amoxicillin is an antibiotic, amongst other widespread misconceptions.
3. Nearly half of respondents accessed antibiotics without prescriptions and 44% shared them, supporting university-level education for students about the proper use, effectiveness, and antibiotic resistance issues.
Medicines play a crucial role in healthcare by reducing illness and death from various diseases. However, when misused, they can cause harmful health effects. According to the World Health Organization (WHO), more than half of all medicines worldwide are either prescribed, dispensed, or used incorrectly, and half of the patients do not follow their medication properly. This issue is especially serious with antibiotics because their inappropriate and excessive use leads to antimicrobial resistance, which is rapidly increasing worldwide. Rational use of medicines means that patients receive the right medication, in the correct dose, for an appropriate period, and at the lowest cost to both themselves and the community.1-9
A lack of public knowledge about antibiotics often results in their irrational use, such as taking incorrect doses, stopping treatment early, and self-medicating. These behaviors can cause treatment failure, reduce trust in the healthcare system, and lead to adverse effects, including antibiotic resistance and gastrointestinal problems. Although healthcare providers have an important role in promoting proper use, the knowledge, attitudes, and cultural context of the community also significantly influence how antibiotics are used.10,11
There has been no previous research on the knowledge and attitudes of students toward antibiotics in Afghanistan, making this the first study of its kind in medical students.
Some studies from neighboring countries show similar concerns. For example, a study in Pakistan found many students confused antibiotics with other medicines, and more than half practiced self-medication.12 Similar findings have been reported in Pakistan, where public knowledge about antibiotic use was insufficient and self-medication was common.13 In Iran, nearly 50% of students self-medicate, and only a small percentage understand the connection between improper antibiotic use and resistance.14-16 In other countries like Uzbekistan, Yemen,17 and Saudi Arabia, a large portion of people and university students use antibiotics without prescriptions and have poor knowledge about antibiotic effectiveness and resistance. These findings reveal widespread misconceptions and negative attitudes that contribute to the irrational use of antibiotics.
The WHO recommends raising public awareness and running educational campaigns as simple and effective ways to promote the rational use of antibiotics.9,18 Therefore, this study aims to assess the knowledge, attitudes, and practices related to antibiotic use among students at Kabul University, Afghanistan, to help design appropriate antibiotic education and awareness programs for them.
This cross-sectional study was conducted to assess the knowledge and attitudes of Kabul University students regarding antibiotics. It
included students from 22 faculties, divided into two groups: natural sciences and social sciences. The study took place between September 2019–December 2019.
The study population consisted of Kabul University students in 2019, with a total of 25,000 students that year. The sample size was calculated using Cochran’s formula, considering a p-value of 0.05 based on online data. Ultimately, the final sample included 1,073 students. The sample size for each faculty was determined, and participants were then randomly selected. A stratified random sampling technique was used. Kabul University consists of several faculties, and to ensure representation from all academic disciplines, a specific number of participants were randomly selected from each faculty. Questionnaires were distributed by trained volunteer data collectors to students in different classes and were recollected after completion. The data collected were then entered and analyzed by the research team. All Kabul University students were included, but graduated students were excluded.
The study’s purpose was explained to the respondents by volunteer data collectors. The authors developed an appropriate questionnaire by reviewing those used in previous studies. Originally in English, the questionnaire was translated into Dari, and both versions were provided. Respondents selected their answers from multiplechoice options: ‘Yes’, ‘No’, or ‘Unsure’. The questionnaire included 18 questions divided into three parts: accessibility of antibiotics, attitudes toward antibiotic use, and antibiotic efficacy and resistance.
The questionnaire comprised two sections. The first section collected demographic information including faculty, academic year, gender, age, and place of residence. The
second section contained 17 items assessing knowledge, attitudes, and practices relating to antibiotic use. The instrument was adapted from previously used surveys in Pakistan, China, Türkiye, and Saudi Arabia, and was translated into Dari for clarity. Items covered recognition of antibiotics, sources of recommendation, self-medication, early discontinuation of treatment, preference for injectable formulations, perceptions of antibiotic strength, understanding of resistance, and awareness of correct antibiotic use. All questions were multiplechoice (‘Yes’, ‘No’, or ‘Unsure’). Participation was voluntary, responses were anonymous, and data were used only for research purposes.
The respondents’ answers were analyzed using the χ2 test with SPSS version 25.0 statistical software (IBM, Armonk, New York, USA). P-values ≤0.05 were considered statistically significant. Attitudes and behaviors regarding antibiotic use and resistance were evaluated by counting the correct answers from Parts 2 and 3 of the questionnaire. Each correct answer was given one point, while incorrect or unsure responses received no points. The total scores were then categorized into three groups: poor, moderate, and high.
The study included 1,073 respondents, of whom 764 were from social science faculties and 309 from natural science faculties. The students’ ages were grouped into four categories: under 20 years, 20–25 years, 25–30 years, and over 30 years. Among the participants, 57.5% were male and 42.5% female (Table 1).
The first part of the questionnaire focused on ‘Access to antibiotics’ (Table 2). Frequency tests were performed for both the Faculty of Natural Sciences and the Faculty of Social Sciences to present the distribution of
responses within each group. Additionally, an independent samples t-test was conducted to compare the mean differences between the two faculties.
More than half of the respondents (52.1%) obtained antibiotics through a doctor’s prescription, with a significant difference between the two groups (P=0.000). Meanwhile, 46.8% relied on their own experience without consulting a doctor. Additionally, 81.2% of respondents said they knew what antibiotics are. However, 77.2% admitted to stopping antibiotic treatment once they felt better, and 44.3% reported sharing their antibiotics with others. The full percentages for this section are detailed in Table 3.
Knowledge about antibiotic efficacy and resistance was also assessed (Table 4). Only 24% correctly identified that antibiotics kill bacteria, while others thought antibiotics targeted different types of microorganisms. Also, 42% recognized amoxicillin as an antibiotic. Overall, 63% of respondents had poor knowledge scores related to antibiotic efficacy, use, and resistance. Students from the natural science faculties scored significantly higher than those from social science faculties, with this difference confirmed by an independent samples t test (p=0.000).
There is no previous study related to the knowledge and attitudes of the public and students regarding antibiotics and their usage. The authors’ study is the first of its kind in Afghanistan in medical students. This study of different faculties at Kabul University is based on understanding the awareness of this sector of the community. Proper educational campaigns can be conducted to correct misconceptions and negative attitudes. In this study, the questionnaire was based on those used in previous studies in other countries, such as Pakistan (Karachi), China, Türkiye (Adana), and Saudi Arabia (Riyadh, Jeddah).14,15,19-22
In the authors’ study, 18% of participants did not know or were unsure about antibiotics. This percentage is higher than the findings reported among students in Pakistan (11%), India (4%), and Nigeria (9%),21,23,24 indicating a greater level of unawareness among the authors’ students. This result (17%) is, however, comparable to studies conducted among the general public in Pakistan.25
More than half of the respondents (52.1%) reported using antibiotics based on a doctor’s prescription. While this is encouraging, it is still lower than the 77.8% reported in a study at Riyadh University in Saudi Arabia.20 In the authors’ study, 35.5% of participants stated that they accessed antibiotics through pharmacist advice, highlighting the crucial role pharmacists play in guiding patients toward the rational use of antibiotics. In comparison, pharmacist advice was reported at 44.1% in another study20 and 39.2% in the study by Mouhieddine et al.26
The most common reason for taking antibiotics without a doctor’s prescription was self-experience (46.8%), reflecting a concerning attitude toward medicine, as people tend to rely on their previous experiences and repeatedly self-medicate. Financial problems (13.3%) and suggestions
from family or friends (8.3%) were also cited as reasons. If we combine the 46.8% who used antibiotics based on self-experience with the 8.3% who followed advice from family or friends, more than half of the respondents practiced self-medication. This highlights the widespread tendency toward irrational antibiotic use. Regarding the type of antibiotic, 43% of respondents selected amoxicillin. Further details are presented in Table 2.
In the authors’ study, 77.2% of respondents reported that they stop taking antibiotics once they feel better. This inappropriate practice increases the risk of developing resistant bacterial strains. Unfortunately, this result is even higher than the rate reported among the general public in Pakistan (73.9%).15 Although respondents were generally aware of the emergence of bacterial resistance, the inadequate level of knowledge appears to contribute to this poor behavior. Improving awareness could lead to better antibiotic practices.
Additionally, 44% of respondents admitted to sharing antibiotics, which is another form of self-medication. The authors’ finding is consistent with that of Kerman University in Iran (45.1%), further highlighting the high prevalence of self-medication. This is a critical concern, as it can lead to serious health consequences.16
On a positive note, 69% of respondents reported reading the instructions on antibiotic labels, a higher rate than the 57% observed in a study conducted by Abu-Mostafa et al.20 in Riyadh, Saudi Arabia. However, some participants mentioned that they do not read the labels because the instructions are often written in English. This underscores the importance of ensuring that antibiotic labels are written in the native language of each country, which is the responsibility of the manufacturing companies. Regarding dosage forms, 35% of respondents expressed a preference for injectable antibiotics over oral
I normally stop taking antibiotics when I start feeling well
Do you share antibiotics with each other?
I usually read the instructions label of
Injection is better than tablets and other oral routes
A strong antibiotic is better than a weak one
If the doctor prescribes an antibiotic, I will try to guide him to the antibiotic I prefer
If your antibiotic is not effective
Reject antibiotic
to your doctor
ones. This reflects a misconception, as many believe the injectable form is more effective. In reality, oral antibiotics are generally safer and more convenient. The misuse of injections can increase the risk of infections
transmitted through syringes and may result in irreversible adverse effects. Encouragingly, 39% of respondents reported that they never attempt to guide doctors toward prescribing a specific antibiotic, instead relying on the
Table 4: Antibiotics efficacy and resistance.
Not completing the full course of
physician’s judgment. This is similar to the 37% observed in Riyadh by Neda et al.20
Finally, 49.9% of respondents believed that more expensive antibiotics are more effective than cheaper ones. However, 30.4% stated that price is not related to efficacy. Research conducted by Naimi et al.27 at the Faculty of Pharmacy, Kabul University, supports the latter view, confirming that cost does not determine the effectiveness of antibiotics.
In the authors’ first question, 81% of respondents reported that they were familiar with antibiotics. However, only 24.2% correctly identified antibiotics as drugs that kill bacteria. Alarmingly, 24.7% believed antibiotics reduce pain, while 36% thought they kill viruses. This confusion between antibiotics and painkillers may lead to inappropriate drug choices when patients suffer from pain or infection. For example, 43% of respondents selected
amoxicillin as an antibiotic, which shows that while many know the name of an antibiotic, misconceptions and poor attitudes toward its use remain widespread.
Antibiotic resistance is recognized as a major public health issue. In the authors’ study, 54% of respondents believed that humans become resistant to antibiotics, whereas only 35% correctly recognized that bacteria develop resistance. This finding indicates that more than half of the respondents hold misconceptions. Furthermore, 47% agreed that antibiotic overuse contributes to resistance, which is lower than the 61.8% reported among the general public in Pakistan.15
Regarding adherence, 63% of respondents understood that not completing the full course of antibiotics may cause resistance, a result similar to that of a study among students in Riyadh (64%).20 Overall, the authors’ findings suggest that knowledge about antibiotic resistance among their participants is inadequate compared to the seriousness of the issue in this region.
Overall, 63% of respondents demonstrated poor knowledge, 33% had moderate knowledge, and only 4% showed good knowledge. Participants from science
References
1. World Health Organization (WHO). Progress in the rational use of medicines. Available at: www.who.int/ publications/i/item/wha-60.16. Last accessed: 18 November 2025.v
2. World Health Organization (WHO). The World Medicines Situation 2011: Access to controlled medicines, 3rd edition. 11 April 2011. Available at: https://www. who.int/publications/i/item/WHOEMP-MIE-2011-2.4. Last accessed: 18 November 2025.
3. Apisarnthanarak A, Mundy LM. Correlation of antibiotic use and antimicrobial resistance in Pratumthani, Thailand, 2000 to 2006. Am J Infect Control. 2008;36(9):681-2.
faculties scored significantly higher in knowledge compared to those from nonscience backgrounds. This difference can be attributed to the greater exposure of students in faculties such as Pharmacy and Biology, where education provides them with a deeper understanding of microorganisms and related subjects.
According to the results of this study, Kabul University students exhibited a negative attitude toward antibiotic use, marked by many misconceptions and poor knowledge. Therefore, it is essential to conduct awareness campaigns to promote the rational use of antibiotics. As the first study of its kind in Afghanistan involving medical students, the authors recommend that similar research be carried out in other educational settings, such as schools, to further enrich our understanding. Additionally, community awareness campaigns should be launched at the start of each university semester to educate students about the appropriate use of antibiotics and the risks of resistance. Pharmacists and other healthcare professionals should actively participate in patient education. Increasing understanding of complications like antibiotic resistance may motivate individuals to change harmful behaviors.
4. Holloway KA et al. The impact of WHO essential medicines policies on inappropriate use of antibiotics. PLoS One. 2016;11(3):e0152020.
5. World Health Organization (WHO). Medicines use in primary care in developing and transitional countries: Fact book summarizing results from studies reported between 1990 and 2006. 2009. Available at: https://iris. who.int/items/81135ff6-dc8b-4d659ff7-303c840971cc. Last accessed: 18 November 2025.
6. R. Wise et al. Antimicrobial resistance. Is a major threat to public health. BMJ. 1998;317(7159):609-10.
7. Gyssens IC. Quality measures of antimicrobial drug use. Int J Antimicrob Agents. 2001;17(1):9-19.
8. Management Science for Health. Encouraging appropriate medicien use by consumers," in Managing Access to Medicines and Health Technologies, Arlington, Management Science for Health. 2012. Available at:https://msh. org/wp-content/uploads/2013/04/ mds3-ch33-consumeruse-mar2012.pdf. Last accessed: 18 November 2025.
9. World Health Organization (WHO). Conference of experts on the rational use of drugs: report by the Director-General. Part III. 1986. Available at: https://iris.who.int/ handle/10665/162008. Last accessed: 18 November 2025.
10. C. Ochoa et al. Assessment of antibiotic prescription in acute respiratory infections in adults. The Spanish Study Group on Antibiotic Treatments. J Infect. 2000;41(1): 73-83.
11. J. Pechère. Patients' interviews and misuse of antibiotics. Clin Infect Dis. 2001;33(Suppl 3):S170-3.
12. Gillani AH et al. Antibiotic selfmedication among non-medical university students in Punjab, Pakistan: A cross-sectional survey. Int J Environ Res Public Health. 2017;14(10):1152.
13. Pavydė E et al. Public knowledge, beliefs and behavior on antibiotic use and self-medication in Lithuania. Int J Environ Res Public Health. 2015;12(6):7002-16.
14. S. J. Shah et al. Self-medication with antibiotics among non-medical university students of Karachi: a crosssectional study. BMC Pharmacol Toxicol. 2014;15:74.
15. Akhund R et al. Knowledge and Attitude of general Pakistani population towards antibiotic resistance. Cureus. 2019;11(3):e4266.
16. Zardosht M et al. Prevalence and causes of self medication among
medical students of Kerman University of Medical Sciences, Kerman, Iran. Global Journal of Health Science. 2016;8(11):150-9.
17. Belkina T et al. Antibiotic use and knowledge in the community of Yemen, Saudi Arabia, and Uzbekistan. J Infect Dev Ctries. 2014;8(4):424-9.
18. Keyvanara M et al. Rational use and prescription of drugs: a review on WHO's 12 atrategies. Hakim Health Sys Res. 2016;18,(4):294-305.
19. Pinar N et al. Medicine use behaviors of people in the city of Adana, Turkey. TAF Preventive Medicine Bulletin. 2013;12(6):639-50.
20. Abu-Mostafa N A et al. A survey of awareness related to the use of antibiotics for dental issues among non-medical female university students in Riyadh, Saudi Arabia. J Infect Public Health. 2017;10(6):842-8.
21. Limaye D et al. Knowledge, attitude and practices of antibiotic usage among university students from Karachi, Pakistan. Int J Res Med Sci. 2019;7(2):519-25.
22. Pan H et al. Prior knowledge, older age, and higher allowance are risk factors for
self-medication with antibiotics among university students in southern China. PLoS One. 2012;7(7):e41314.
23. Limaye D et al. Knowledge, attitude and practices of antibiotic usage among students from Mumbai University. Inter J Res Med Sci. 2018;6(6):1908-12.
24. Igbeneghu OA. Knowledge and practices in the use of antibiotics among a group of Nigerian university students. Inter J Infection Control. 2013;DOI:10.3396/ijic.v9i1.10539.
25. Naseem S et al. Knowledge about antibiotic use amongst the public: a cross sectional study in Karachi. Infectious Dis J Pak. 2016;25(3):49-54.
26. Mouhieddine TH et al. Assessing the Lebanese population for their knowledge, attitudes and practices of antibiotic usage. J Infect Public Health. 2014;8(1):20-31.
27. Naimi H et al. Assessment of the price-efficacy relationship for multiple brands of ceftriaxone sodium in Kabul: a cross-sectional study. BMC Res Notes. 2016;DOI:10.1186/s13104.
If you like it,why not share it...

Share with a colleague today